Is Cardiovascular Risk being Effectively Monitored and Managed in Patients with Inflammatory Polyarthritis?
by Olivia Fraser[1] and Tarnya Marshall,[2] School of Medicine, Health Policy and Practice, University of East Anglia
Abstract
Objective
To investigate assessment and management of cardiovascular disease (CVD) risk in two cohorts of inflammatory polyarthritis (IP) patients (on biologic therapy (infliximab) or traditional disease modifying anti-rheumatic drugs (DMARDs)) and to compare findings with NICE guidance on hypertension, statin therapy and secondary prevention of CVD.
Hypotheses
- CVD risk in IP patients is not consistently monitored or managed in line with NICE guidance.
- IP patients receiving biologic (infliximab) therapy have CVD risk factors more regularly monitored and appropriately managed than those on traditional DMARDs.
Methods
Demographics, disease severity data, cardiovascular risk factors, recall of lifestyle advice provision and cardiovascular drug history were obtained from interviews, medical notes, electronic records and blood pressure measurement.
Results
135 participants were recruited from a regional teaching hospital; 90 on infliximab, 45 on traditional DMARDs. The DMARD-alone cohort were less likely to have undergone a lipid profile in the last two years than the infliximab cohort (57.8% vs 85.6%, p=0.0003). Most participants did not recall receiving lifestyle advice; 22.2% (infliximab cohort) vs 20.0% (DMARD cohort) reported receiving diet or weight loss advice.
Conclusions
IP patients on DMARD-alone therapy are less likely to have lipid profiles monitored regularly. Lifestyle advice is poorly provided to IP patients.
Key points
- Patients with inflammatory polyarthritis who are on DMARD-alone therapy have lipid profiles less rigorously monitored than those receiving biologic therapy.
- Lifestyle advice concerning methods of reducing cardiovascular risk is inadequately provided to patients with inflammatory polyarthritis, this may be due to lack of clarity regarding whether the responsibility lies with primary or secondary care health professionals.
Sources of support
Jean Shanks Foundation (educational grant).
Keywords: Inflammatory polyarthritis, cardiovascular risk factors, NICE guidelines
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory condition primarily targetting the synovial tissue of peripheral joints (Gonzalez-Gay et al., 2006; Voskuyl, 2006).
Patients with RA have a life expectancy of two to eighteen years less than the general population (Van Doornum et al., 2002; Walberg-Jonsson et al., 1999). In the majority of RA patients, death is a result of a cardiovascular event (myocardial infarction (MI), transient ischaemic attack (TIA) or stroke) (Goodson et al., 2005; Solomon et al., 2003; Sattar et al., 2003) and a recent meta-analysis found that cardiovascular disease (CVD) mortality is increased by 50% in RA (Aviña-Zubieta et al., 2008). An increased risk of CVD has more recently been associated with other inflammatory rheumatic diseases such as psoriatic arthritis and ankylosing spondylitis (Han et al., 2006; Tam et al., 2008).
There are three possible mechanisms for the increased risk of CVD in those with IP: increased prevalence of established cardiovascular risk factors, lack of risk factor modification, or a direct effect of the disease due to the underlying inflammatory process (van Halm et al., 2006).
Traditional CVD risk factors such as a history of smoking (Symmons et al., 1997; Hutchinson et al., 2001) hyperlipidaemia (Hall and Dalbeth, 2005), hypertension (Goodson et al., 2005; Panoulas et al., 2007), thrombotic variables and sedentary lifestyle (McEntegart et al., 2001) are more common amongst RA patients than in the general population.
However, CVD prevalence in the RA population is higher than can be accounted for by increased prevalence of traditional CVD risk factors alone. It appears that the inflammatory nature of IP leads to an accelerated process of atheromatous plaque formation (Douglas et al., 2006; Del Rincon et al., 2001). For this reason the need to control RA disease activity through the use of traditional disease modifying anti-rheumatic drugs (DMARDs) and newer biologic agents such as those targeting tumour necrosis factor (e.g. infliximab) is advocated to reduce CVD risk as well as to provide symptomatic relief.
Given the established link between RA and CVD, attention is increasingly focused on risk reduction using pharmacological and lifestyle interventions. The National Institute for Health and Clinical Excellence (NICE) produces best practice and evidence based guidelines with the aim of promoting good health and preventing and treating ill health in the general population.
NICE guidelines addressing management of hypertension (NICE, 2006a) recommend that anti-hypertensive therapy should be offered to those with a persistently high blood pressure (BP) of ≥160/110 mmHg; or those at 10-year cardiovascular risk of ≥20% (or with existing CVD or target organ damage) with persistent BP of >140/90 mmHg. These guidelines also recommend that lifestyle advice be given to patients being treated or under assessment for hypertension.
NICE guidance concerning statin use (NICE, 2006b) state that provision of statin therapy should be for adults with clinical evidence of CVD or adults with ≥20% 10-year risk of developing CVD.
Guidelines covering secondary prevention following a cardiovascular event have also been published by NICE (NICE, 2007b). This guidance advocates regular physical activity, smoking cessation where appropriate, and dietary advice as well as pharmacological therapy with angiotensin converting enzyme inhibitors, aspirin, β-blockers and statins.
Although there have been no NICE guidelines published specifically concerning aspirin use in CVD, immediate and long-term treatment with aspirin following an MI is recommended (NICE, 2007b). In addition, primary and secondary prevention studies have demonstrated that aspirin reduces the risk of cardiovascular events by roughly 25% (Hall and Dalbeth, 2005; Sanmuganathan et al., 2001) and may be appropriate for RA patients with high CVD risk who have no past medical history (PMH) of CVD (Pham et al., 2006).
It has been demonstrated that increased knowledge of CVD risk factors is correlated with improved concordance with medication, successful and sustained lifestyle modification and improvement in individual CVD risk factors (John et al., 2007). Following an MI, advice concerning lifestyle changes (including dietary modification, physical activity and smoking cessation) is also advocated by NICE (NICE, 2006a). Primary care personnel are particularly well placed to give lifestyle advice concerning CVD risk reduction, which includes smoking cessation, exercise advice, weight reduction and diet advice (Symmons and Bruce, 2006).
| Risk factor | Intervention |
|---|---|
| Primary prevention | |
| Hypertension |
|
| Plasma cholesterol |
|
| Secondary prevention |
|
Table 1: summary of NICE guidance regarding management of cardiovascular risk factors
An individual's cardiovascular risk can be estimated using a cardiovascular risk assessor (CVRA). A CVRA uses CVD risk factor data (such as age, sex, smoking status, BP, total cholesterol and high-density lipoprotein (HDL) cholesterol) to produce an absolute and relative risk score reflecting risk of a coronary heart disease-related event over a ten-year period (Sheridan et al., 2003). The absolute cardiovascular risk reflects an individual's risk expressed as a percentage, and is classified as low (<10% risk of an event in ten years), intermediate (10-20%) and high (>20%) (Ford et al., 2004). Relative risk score reflects an individual's chance of developing CVD compared to an age-matched individual with no CVD risk factors. One such CVRA is the Manchester Joint Societies CVRA (Manchester 2006 Joint British Society's Cardiovascular Risk Assessor); this CVRA is not appropriate for use in those with a PMH of a cardiovascular event and/or a diagnosis of diabetes mellitus (DM).
A number of studies have attempted to establish specific standards for addressing cardiovascular risk in RA patients and the major strategies for risk reduction are regarded as correcting traditional cardiac risk factors alongside tight control of disease activity (Hall and Dalbeth, 2005; Van Doornum et al., 2006; Symmons and Bruce, 2006; Pham et al., 2006). It has also been recommended that screening for cardiovascular risk factors should be carried out annually in all RA patients (Symmons and Bruce, 2006).
Despite the increased CVD risk burden in those with inflammatory arthritis, it is not clear whether risk factor modification is offered or heeded by patients. In particular it is not known whether CVD risk factors are being conscientiously monitored and managed in individuals with RA or other forms of IP.
The aims of this study were:
- To assess whether IP patients' CVD risk factors are effectively monitored and managed in primary care, and in particular whether hypertension and hypercholesterolaemia are addressed in accordance with NICE guidelines.
- To compare the management of cardiovascular risk factors in two cohorts of IP patients, the first with more severe disease receiving biologic therapy (which requires closer and more frequent monitoring of patients) and the second receiving traditional DMARD-alone therapy.
Hypotheses
Cardiovascular risk factors in those with IP are not consistently monitored or addressed in accordance with NICE guidance.
IP patients receiving biologic therapy (infliximab) have their risk factors more regularly monitored and appropriately managed than those on DMARDs.
Materials and Methods
The study recruited 135 patients receiving treatment for IP (rheumatoid arthritis, juvenile chronic arthritis or inflammatory psoriatic arthritis) at the Norfolk and Norwich University Hospital (NNUH).
Participants were drawn from two distinct cohorts – those receiving four-six weekly infliximab infusions at the NNUH Rheumatology Day Unit and those receiving DMARD therapy and attending six-monthly nurse practitioner (NP) follow-up appointments.
First Cohort
Participants were identified from a list of patients receiving infliximab for treatment of IP as day cases in October 2007. Participants consented to interview and/or access to medical records.
The following data were collected at interview: age, sex, height, date of IP diagnosis, duration of biologic therapy, smoking history (positive smoking history led to further questioning in order to calculate pack years), co-existing diabetes mellitus, cardiovascular drug history (specifically cholesterol-lowering, anti-hypertensive medication or aspirin), personal history of CVD (MI, stroke, TIA, angina or heart failure); family history of CVD (MI, stroke, TIA, angioplasty or coronary bypass grafting in a male first degree relative <55 years or a female first degree relative <65 years); and recall of lifestyle advice provision – smoking cessation, healthy eating, weight loss or exercise advice given with the aim of reducing CVD risk.
Participants' hospital notes and computerised laboratory records were examined to determine: the median blood pressure reading from the last three attendances; most recent weight; most recent lipid profile (LP) (total cholesterol, high density lipoprotein and triglycerides); date of test (classified as in the past two years or longer; a two-year cut-off was decided upon due to the current NICE guidance recommendation of a five-year assessment and IP-specific publications advocating yearly LP assessment); fasting glucose level; and evidence of left ventricular hypertrophy (LVH) on ECG or echocardiogram. The three most recent CRP and ESR values, most recent disease activity score (DAS28), rheumatoid factor positivity and presence of erosions on x-ray were also collected to determine disease severity. Medical records were used to verify responses to self-reported details, including documentation of healthcare professionals providing cardiovascular risk-reducing lifestyle advice.
Second Cohort
The second cohort was included in order to gain insight into the monitoring and management of cardiovascular risk factors amongst a distinct group of IP patients who were receiving DMARD-alone therapy and to compare findings with those on biologic therapy. These patients were seen at six-monthly periods by a consultant rheumatologist or nurse practitioner in the Rheumatology Department. It was therefore hypothesized that their cardiovascular risk factors would be less rigorously monitored and managed due to their less frequent hospital attendances, providing fewer opportunities for assessment of risk factors as well as less correspondence to GPs advocating cardiovascular risk reduction. Information sheets were posted to 133 patients identified from clinic lists for NP DMARD follow-up appointments between 12 May and 19 June 2008. This method ensured participants had sufficient time to consider participation and that confidentiality was preserved (medical records were not accessed prior to consent being obtained).
Two BP readings were collected for each participant. The data collected in this cohort was identical to that for the first cohort but included duration of DMARD therapy.
Each participant's general practitioner (GP) received a letter informing them of their patient's enrolment in the study.
Data Analysis
Participants' ten-year cardiovascular risk scores were calculated using the Manchester Joint Societies CVRA (Manchester 2006 Joint British Society's Cardiovascular Risk Assessor).
Following data analysis, GPs received a letter providing details of their patient's cardiovascular risk score, average blood pressure, lipid profile and comparison of data with NICE guidelines. This ensured that where cardiovascular risk factors were suitable for modification, GPs were informed and could intervene appropriately.
Where LP data was missing and therefore calculation of cardiovascular risk impossible, participants' GPs received a letter requesting missing data which may be available to them but which was not evident within the hospital notes or electronic system to be sent to the chief investigator.
Statistical analysis was carried out using SPSS 16.0 for Windows. Independent sample t-tests were used to compare continuous data and Chi-Square or Fisher's exact tests were used to compare categorical data. Results with a p-value of <0.05 were considered statistically significant.
Ethical Approval
Ethical approval was provided by the Suffolk Research Ethics Committee and the East Norfolk and Waveney Research Governance Committee. A successful application for a substantial amendment allowing for inclusion of the second cohort was submitted to the ethics and research governance committees in February 2008.
Results
Demographic and IP Data
107 IP patients receiving infliximab were invited to take part and 90 (84.1%) consented (88 to interview and medical record examination, two to medical record examination only). 45 patients with IP attending a NP DMARD follow-up appointment within the study period were eligible for inclusion in the study and consented to participation (33.8% of the 133 patients who had been posted information sheets). Of these, 43 consented to interview and medical record examination, two to medical record examination only.
| Demographics | Infliximab cohort (n=90) |
DMARD cohort (n=45) |
All participants (n=135) |
|---|---|---|---|
| Age | 59 (±12.70) | 63 (±13.41) | 60 (±13.01) |
| Sex Male Female |
22 (24.0) 68 (76.0) |
9 (20.0) 36 (80.0) |
31 (23.0) 104 (77.0) |
| Ethnicity White, British White, Other Asian/Asian British Black, any other background |
88 (97.8) 2 (2.2) 0 0 |
42 (93.3) 0 2 (4.4) 1 (2.2) |
130 (96.3) 2 (1.5) 2 (1.5) 1 (0.7) |
Table 2: Demographic data
Values are mean (± standard deviation) or n (%)
Demographic data are displayed in Table 2. Participants in the two cohorts were similar in age, sex and ethnicity. Age ranged from 21 to 88 and average age for all participants was 60 years. Roughly one third of participants were male and two thirds female; almost all were Caucasian. Table 3 demonstrates IP characteristics. The majority of participants had a diagnosis of seropositive RA and a total of 17 participants had been diagnosed with psoriatic arthritis; the differences in type of IP in each cohort were statistically significant (p=0.010). There were also statistically significant differences between disease duration, treatment duration, DAS-28 and evidence of erosions on x-ray in each cohort.
| IP characteristics | Infliximab cohort (n=90) |
DMARD cohort (n=45) |
|---|---|---|
| Diagnosis RA RF Positive RA RF Negative RF unavailable Psoriatic arthritis Juvenile chronic arthritis |
61 (67.8) 13 (14.4) 0 14 (15.6) 2 (2.2) |
24 (53.3) 18 (40.0) 1 (2.2) 3 (6.7) 0 |
| Disease duration (years) Treatment duration (years) |
15.6 (±1.8) 3.4 (±0.6) |
8.3 (±1.9) 4.86 (± 0.99) |
| Disease activity CRP (mg/l) ESR (mm/hour) Disease activity score-28 DAS-28 over 5.1 Erosions on x-ray |
17 (± 4.6) 40 (± 5.1) 4.39 (± 0.3) 29 (32.2) 69 (76.7) |
12 (±3.68) 32 (± 5.47) 4.16 (± 0.39) 10 (22.2) 16 (35.6) |
Table 3: Inflammatory polyarthritis characteristics
Values are mean (± 90% confidence interval) or n (%)
RF : Rheumatoid factor
Cardiovascular Risk Factors and Previous Cardiovascular Events
The prevalence of cardiovascular risk factors and previous cardiovascular events are displayed in Table 4. Twenty-one of the participants had a personal history of a cardiovascular event and a quarter of all participants had a positive family history of CVD. There was no statistically significant difference in prevalence of DM, dyslipidaemia or LP values when the cohorts were compared.
Thirty participants had no record of a test having been conducted in the past two years (data on LP date were missing for two participants). Statistically, a significantly smaller proportion of the DMARD cohort had undergone a LP in the previous two years (p=0.0003).
Equal proportions of both groups were hypertensive but a greater proportion of the DMARD group had isolated systolic hypertension, although this did not reach statistical significance. The DMARD group had higher average diastolic BP than the infliximab group (p=0.004). One fifth of the participants were current smokers; the DMARD cohort had a greater proportion of ex-smokers but the differences in smoking patterns between the cohorts was not statistically significant, nor were average pack years.
BMI ranged from 16 to 52, although there was no statistically significant difference between the groups.
| Cardiovascular Risk Factor | Infliximab cohort (n=90) |
DMARD cohort (n=45) |
p values | All participants (n=135) |
|---|---|---|---|---|
| History of CVD MI TIA Stroke Angina Stroke, MI and TIAs Heart failure |
14 (15.5) 6 (6.7) 1 (1.1) 2 (2.2) 5 (5.5) 0 0 |
7 (15.5) 2 (4.4) 0 0 3 (6.7) 1 (2.2) 1 (2.2) |
1.0 |
21 (15.6) 8 (5.9) 1 (0.7) 2 (1.5) 8 (5.9) 1 (0.7) 1 (0.7) |
| Positive family history of CVD | 22 (24.4) | 12 (26.7) | 0.779 | 34 (25.2) |
| Diabetes mellitus Type 1 Type 2 |
2 (2.2) 4 (4.4) |
0 3 (6.7) |
0.314 0.583 |
2 (1.5) 7 (5.2) |
| Lipid profile Dyslipidaemia (total:HDL ratio>6) Total cholesterol (n=124) Total:HDL ratio (n=115) |
4 (4.4) 5.2 (±0.2) 3.9 (±0.2) |
2 (4.4) 5.2 (±0.3) 4.0 (±0.4) |
1.000 0.721 0.809 |
6 (4.4) 5.2 (±0.1) 3.9 (±0.2) |
| Lipid profile in last 2 years | 77 (85.6) | 26 (57.8) | 0.0003 | 103 (76.3) |
| Blood pressure (BP) Average systolic BP Average diastolic BP Systolic BP >140 mmHg Hypertension (BP>140/90mmHg) |
129 (±2.9) 74 (±1.7) 20 (22.2) 6 (6.7) |
134 (±4.2) 80 (±2.7) 14 (31.1) 3 (6.7) |
0.131 0.004 0.262 1.000 |
131 (±2.4) 76 (±1.4) 34 (25.2) 9 (6.7) |
| Smoking status Never smoked Ex-smokers Current smokers No interview, no positive smoking history in notes Average pack years |
40 (44.4) 26 (28.9) 22 (24.4) 2 (2.2) 7.5 (±2.0) |
19 (42.2) 19 (42.2) 5 (11.8) 2 (4.4) 12.2 (±4.9) |
0.187 0.154 |
59 (43.7) 45 (33.3) 27 (20.0) 4 (29.6) 9.08 (±2.1) |
| Body Mass Index (BMI) Average BMI Overweight (25.00-29.99 kg/m2) Obese (≥30.00 kg/m2) |
26.7 (±1.1) 27 (30) 25 (27.8) |
28.0 (±1.4) 15 (33.3) 18 (40.0) |
0.268 0.661 0.137 |
27.0 (±0.9) 42 (31.1) 43 (31.9) |
| LVH (on ECG or echocardiogram) | 8 (8.9) | 5 (11.1) | 0.680 | 13 (9.6) |
Table 4: Cardiovascular risk factors and history of CVD
Values are mean (± 90% confidence interval) or n (%)
Calculated Ten-Year Cardiovascular Risk Scores
Participants' ten-year cardiovascular risk (CVR) scores are demonstrated in Table 5. CVD absolute and relative risk scores were calculated for 92 participants; the remaining 43 participants had missing LP data and/or a PMH of a cardiovascular event or diagnosis of DM, rendering use of the CVRA unsuitable.
| Ten-year CVR for those without PMH or DM | Infliximab cohort (n=64) |
DMARD cohort (n=28) |
p values | All participants (n=92) |
|---|---|---|---|---|
| Absolute cardiovascular risk (%) | 12.3 (± 2.1) | 14.07 (±2.6) | 0.429 | 12.87 (±1.64) |
| Relative cardiovascular risk | 37.2 (± 6.2) | 43.4 (±9.6) | 0.370 | 39.11 (±5.21) |
| Absolute CVR ≥20% | 12 (18.8) | 6 (21.4) | 0.766 | 18 (19.6) |
Table 5: Ten-year cardiovascular risk scores for those without PMH of CVD or DM
Values are mean (± 90% confidence interval) or n(%)
A total of 18 participants with no PMH of CVD or a diagnosis of DM had high absolute cardiovascular risk scores (≥20%). Absolute and relative cardiovascular risk scores were higher in the DMARD cohort, and there was a higher proportion of the DMARD cohort with a high CVR, these differences between the cohorts were not statistically significant.
Modification of Cardiovascular Risk
Table 6 displays data concerning modification of cardiovascular risk in participants with regards to cardiovascular pharmacotherapy and provision of lifestyle advice to participants.
| CVR Modification | Infliximab cohort (n=90) |
DMARD cohort (n=45) |
p value |
|---|---|---|---|
| Antihypertensive use 1 anti-hypertensive 2 anti-hypertensives 3 or more anti-hypertensives |
31 15 12 4 |
20 10 5 5 |
0.591 |
| Aspirin use | 15 | 6 | 0.075 |
| Statin use | 24 | 10 | 0.492 |
| Lifestyle risk factors and modification | |||
| Ever smokers | 49 (54.4) | 24 (53.3) | 0.903 |
| Smoking cessation advice received | 15 (16.7) | 10 (22.2) | 0.433 |
| Body mass classification Overweight (25.00-29.99 kg/m2) Obese (≥30.00 kg/m2) |
27 (30) 25 (27.8) |
15 (33.3) 18 (40.0) |
0.661 0.137 |
| Diet or weight loss advice received | 20 (22.2) | 9 (20.0) | 0.767 |
| Exercise advice received | 11 (12.2) | 8 (17.8) | 0.382 |
Table 6: Pharmacological and lifestyle modification of cardiovascular risk
Values are n(%)
Pharmacological Intervention
In total, 51 of the participants (37.8%) were taking anti-hypertensive medication (angiotensin converting enzyme inhibitors, beta blockers, calcium channel blockers, diuretics, alpha blockers or potassium channel agonists). Of those on anti-hypertensives, four had blood pressures >140/90mmHg and 14 had isolated systolic blood pressure >140mmHg.
Of the nine cases of hypertension (BP >140/90) identified (six in the infliximab group and three in the DMARD group), four were taking anti-hypertensive medication. Four of these nine participants with hypertension had absolute cardiovascular risk scores ≥20% and two had incalculable cardiovascular risks due to lacking LP data. Two had received any form of lifestyle advice concerning CVR reduction.
Of the six participants with dyslipidaemia, one was on cholesterol-reducing treatment and one had previously been on cholesterol-lowering treatment (both of these participants were in the infliximab cohort). Of the four new cases, there were two in each cohort.
There was no statistically significant difference in the proportion of each cohort taking CVD risk-reducing medication.
Provision of Lifestyle Advice
Table 6 and Figure 1 demonstrate the prevalence of CVD lifestyle risk factors in each cohort and the proportion of those participants who had received relevant advice.
The majority of participants in each cohort had not received any lifestyle advice concerning methods of reducing CVD risk. The proportion of ever smokers in the DMARD cohort who had received smoking cessation advice was higher (22.2% vs 16.7%), however a greater proportion of the infliximab cohort had received any diet or weight loss advice (22.2% vs 20%), despite there being a greater prevalence of overweight and obesity in the DMARD group. However, none of these differences in provision of lifestyle advice between the two cohorts reached statistical significance.
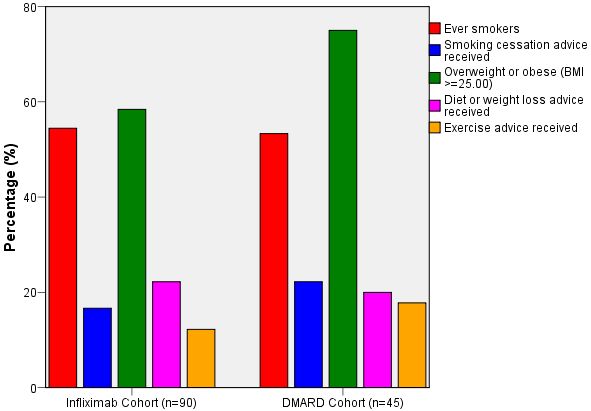
Figure 1: Prevalence of lifestyle variables and recall of lifestyle advice
Primary Prevention
Of those with an absolute CVR≥20% but with no PMH of CVD, there was no significant difference between the two cohorts regarding use of anti-hypertensives (five in the infliximab group, five in the DMARD group; p=0.152), aspirin (zero in each group), statins (five in the infliximab cohort, zero in the DMARD group; p=0.114) or provision of lifestyle advice (four infliximab participants vs two DMARD participants; p=1.000). Of those not receiving anti-hypertensive medication, two (both in the infliximab cohort) were hypertensive.
Secondary Prevention
Of those with a PMH of CVD, 14 were in the infliximab and seven in the DMARD cohort. Just over three quarters of these recalled receiving lifestyle advice. There were no statistically significant differences between the provision of CVR-reducing medication or lifestyle advice between these two subgroups within the cohorts. Two of the participants with a PMH of CVD had no record of an LP in the last two years (both were in the DMARD cohort). Only one participant had a total:HDL cholesterol of over six and this participant was already receiving statin therapy.
| Management of those with PMH of CVD | Infliximab cohort (n=14) |
DMARD cohort (n=7) |
All participants with PMH of CVD (n=21) | p values |
|---|---|---|---|---|
| Anti-hypertensives | 12 (85.7) | 6 (85.7) | 18 (85.7) | 0.687 |
| Aspirin | 12 (85.7) | 4 (57.1) | 16 (76.2) | 0.325 |
| Statins | 13 (92.9) | 6 (85.7) | 19 (90.5) | 0.283 |
| Lifestyle advice – any lifestyle advice Smoking cessation advice Diet/weight loss advice Exercise advice |
10 (71.4) 6 (42.9) 8 (57.1) 4 (28.6) |
6 (85.7) 4 (57.1) 3 (42.9) 3 (42.9) |
16 (76.2) 10 (47.6) 11 (52.4) 7 (33.3) |
0.624 0.659 0.659 0.638 |
Table 7: Management of those with PMH of CVD
Values are n(%)
Discussion
To our knowledge, this is the first study to compare directly management of cardiovascular risk in IP patients with NICE guidelines and to compare monitoring and management of CVD risk in two distinct cohorts of IP patients.
Previous studies have highlighted the need to monitor rigorously and to manage CVD risk factors amongst IP patients and have made recommendations regarding how this could be done (Hall and Dalbeth, 2005; Pham et al., 2006). Although the majority of participants' cardiovascular risk factors were being monitored and managed in accordance with NICE guidelines, this study highlights a disparity in monitoring of lipid profiles between IP patients receiving biologic therapy and those receiving DMARD-alone therapy.
The statistically significant difference between recent LP testing in the cohorts may be due to the requirements for monitoring of those on biologic drug therapy due to their relatively recent introduction for use in IP, the need to assess their efficacy and the potential for adverse effects. For these reasons, patients receiving infliximab are seen by a doctor at every infusion appointment and at least every six months by a NP and/or consultant rheumatologist, providing increased opportunities for LP assessment.
In some who had undergone lipid profiling, total cholesterol alone had been measured; this is an inadequate assessment as in RA a low total cholesterol can disguise a low high-density lipoprotein level and high low density lipoprotein and triglyceride levels (Hall and Dalbeth, 2005). However, the small number of new cases reflect that in general where dyslipidaemia had been identified, it had led to pharmacological intervention and/or dietary recommendations.
The increased likelihood of those receiving DMARD-alone therapy to have a higher BP may reflect less frequent monitoring and intervention; however it is not possible to exclude the impact that use of non-steroidal anti-inflammatory drugs or the use of glucocorticoids, both recognised to increase risk of hypertension (Panoulas et al., 2007), may have had amongst this cohort.
There were a large number of p values created through comparison of the subjects' demographic, disease characteristic and cardiovascular disease risk data, which may have resulted in false positive results. However, the statistically significant differences of principal importance (lipid profile in the last two years and diastolic blood pressure) produced p values which were well below the cut-off of 0.05 (0.0003 vs 0.004 respectively) thereby making it less likely that these were false positive findings.
The finding of five participants with high CVR who were not taking statins or aspirin implies that there may be a role for regular screening of CVR amongst IP patients, as advocated in publications concerning CVD risk management in IP (Symmons and Bruce, 2006).
The majority of participants with a PMH of CVD were receiving anti-hypertensives, statins and aspirin as recommended by NICE guidelines on secondary prevention in CVD (National Institute for Health and Clinical Excellence 2007 'MI: secondary prevention'). Those who were not receiving aspirin may have had a contraindication for aspirin therapy which was not ascertained at data collection.
The majority of participants did not recall receiving lifestyle advice concerning reduction of CVD risk, including five of the sub-group who had a PMH of CVD. This may be because it is not clear whose role it is to provide such advice. Cardiovascular risk modification is usually undertaken in primary care, however the importance of addressing CVD risk amongst patients with inflammatory arthritis is a relatively new message. It may therefore be under-recognised, resulting in the responsibility falling upon those working in secondary care where there is less expertise and experience in this area. However, the potential for recall bias amongst these patients cannot be excluded and although evidence for documentation of lifestyle advice provision was assessed in hospital notes, primary care records (where lifestyle advice is more commonly given) were not accessed.
The participants in each cohort were similar in age, ethnicity and sex; the higher proportion of female participants reflects the higher prevalence of IP in females. Almost all participants were Caucasian, reflecting the ethnicity of the surrounding population. The NNUH is a teaching hospital with a catchment area of up to 822,500 people in a predominantly rural area; the population of Norfolk is predominantly Caucasian and older when compared to the remainder of England. However, the regularity of attendances for infliximab infusions, as well as the timing of follow-up for patients on DMARDs is similar nationwide, allowing for similar opportunities for risk factor assessment and modification, this suggests that the study's findings are generalisable to other areas.
The majority of participants had seropositive RA and there were significant differences between IP diagnoses in the two groups. However, those with a diagnosis of psoriatic arthritis or juvenile chronic arthritis receiving infliximab therapy do so because their disease severity matches a picture of severe RA, therefore the boundaries of diagnoses between the three conditions is often not clear-cut.
The only statistically significant difference between IP severity was concerning the presence of erosions on x-ray. The infliximab cohort also had a longer disease duration but shorter therapy duration, reflecting the criteria for initiation of biologic therapy amongst IP patients which relate to disease severity and a failure to respond to treatment with traditional DMARDs (NICE, 2007b).
The data collected from these two cohorts gives insight into how CVD risk is currently being managed in IP patients as well as within these two sub-groups. The participants receiving DMARDs were less easily accessible but their presence in the study is of importance as the majority of IP patients have their CVD risk managed in the community. The DMARD cohort was half the size of the infliximab cohort, a reflection of the difficulty in accessing this sub-group, as well as time limitations. Having equal numbers in each cohort would have enhanced the comparison of the two groups and a larger sample size from a broader demographic would have increased the potential for generalising the findings.
In conclusion, this study demonstrates that in the majority of individuals with IP, monitoring and management of CVD risk is conducted in accordance with NICE guidelines. However, clear and comprehensive lifestyle advice concerning CVD risk reduction is not being provided to patients and those receiving DMARD-alone therapy do not have their cholesterol levels as rigorously monitored as those on infliximab therapy.
These findings suggest that further investigation through a larger trial is necessary, possibly involving participants receiving other biologic therapies, particularly those requiring less frequent hospital attendances than infliximab therapy. Also, there is a need for clear guidelines concerning which healthcare professionals are responsible for assessment and management of CVD risk factors in IP if the burden of CVD amongst these individuals is to be reduced.
List of Illustrations
Figure 1: Prevalence of lifestyle variables and recall of lifestyle advice
List of Tables
Table 1: Summary of NICE guidance regarding management of cardiovascular risk factors
Table 2: Demographic data
Table 3: Inflammatory polyarthritis characteristics
Table 4: Cardiovascular risk factors and history of CVD
Table 5: Ten-year cardiovascular risk scores for those without PMH of CVD or DM
Table 6: Pharmacological and lifestyle modification of cardiovascular risk
Table 7: Management of those with PMH of CVD
Notes
[1] Following her study at the University of East Anglia Olivia Fraser is now a foundation year 2 doctor in London.
[2] Dr Tarnya Marshall is a Consultant Rheumatologist at the Norfolk and Norwich University Hospital.
References
Aviña-Zubieta, J. A., H. K. Choi, M. Sadatsafavi, M. Etminan, J. M. Esdaile and D. Lacaille (2008), 'Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies', Arthritis & Rheumatism, 59 (12), 1690-97
Del Rincon, I. D., K. Williams, M. P. Stern, G. L. Freeman and A. Escalante (2001), 'High incidence of cardiovascular events in rheumatoid arthritis cohort not explained by traditional cardiac risk factors', Arthritis & Rheumatism 44 (12), 2737-45
Douglas, K. M. J., A. V. Pace, G. J. Treharne, A. Saratzis, P. Nightingale, N. Erb, M. J. Banks and G.D. Kitas (2006), 'Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome', Annals of the Rheumatic Diseases, 65, 348-53
Ford, E. S., W. H. Giles and A. H. Mokdad (2004), 'The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III', Journal of the American College of Cardiology, 43, 1791-96
Gonzalez-Gay, M. A., C. Gonzalez-Juanatey, J. A. Miranda-Filloy, C. Garcia-Porrua, J. Llorca and J. Martin (2006) 'Cardiovascular disease in rheumatoid arthritis', Biomedicine & Pharmacotherapy, 60, 673-77
Goodson, N., J. Marks, M. Lung and D. Symmons (2005), 'Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s', Annals of the Rheumatic Diseases, 64, 1595-1601
Hall, F.C. and N. Dalbeth (2005), 'Disease modification and cardiovascular reduction: two sides of the same coin?', Rheumatology, 44, 1473-82
Han C., D.W. Robinson, M. V. Hackett, L. C. Paramore, K. H. Fraemore and M. V. Bala (2006), 'Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis', Journal of Rheumatology, 33 (11), 2167-72
Hutchinson, D., L. Shepstone, R. Moots, J. T. Lear and M. P. Lynch (2001), 'Heavy cigarette smoking is strongly associated with rheumatoid arthritis, particularly in patients without a family history of rheumatoid arthritis', Annals of the Rheumatic Diseases, 60, 223-27
John, H., E. D. Hale, G. J. Treharne and G. D. Kitas (2007), 'Patient education on cardiovascular aspects of rheumatoid disease: an unmet need', Rheumatology (Editorial), 46 (10), 1513-16
Manchester 2006 Joint British Society's Cardiovascular Risk Assessor – Joint British Society's Cardiovascular risk assessor: Copyright University of Manchester; Heart 2005; 91; sppl v: v1-52
McEntegart, A., H. A. Capell, D. Creran, A. Rumley, M. Woodward and G. D. O. Lowe (2001), 'Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis', Rheumatology, 40, 640-44
National Institute for Health and Clinical Excellence (NICE) (2006a), 'Management of hypertension in adults in primary care' http://www.nice.org.uk/guidance/CG34, accessed 04/02/08
NICE (2006b), 'Statins for the prevention of cardiovascular events' http://www.nice.org.uk/guidance/TA94, accessed 10/07/08
NICE (2007a), 'MI: secondary prevention', http://www.nice.org.uk/CG48, accessed 20/08/08
NICE (2007b), 'Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis' http://www.nice.org.uk/TA130, accessed 11/07/08
Panoulas, V. F., K. M. J. Douglas, H. J. Milionis, A. Stavropoulos-Kalinglou, P. Nightingale, M. D. Kita, A. L. Tselios, G. S. Metsios, M.S. Elisaf and G.D. Kitas (2007), 'Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis', Rheumatology, 46 (9), 1477-82
Pham, T., L. Gossec, A. Constantin, S. Pavy, E. Bruckert, A. Cantagrel, B. Combe, R-M Flipo (2006), 'Cardiovascular risk and rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion', Joint Bone Spine, 73, 379-87
Sanmuganathan, P. S., P. Ghahramani, P. R. Jackson, E. J. Wallis and L. E. Ramsay (2001), 'Aspirin for primary prevention of coronary heart disease; safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials', Heart, 85, 265-71
Sattar, N., D. W. McCarey, H. Capell and I. B. McInnes (2003), 'Explaining how 'high-grade' systemic inflammation accelerates vascular risk in rheumatoid arthritis', Circulation, 108, 2957 – 63
Sheridan, S., M. Pignone and C. Mulrow (2003) 'Framingham-based tools to calculate the global risk of coronary heart disease', Journal of General Internal Medicine, 18, 1039-52
Solomon, D. H., E. W. Karlson, E. B. Rimm, C. C. Cannuscio, L. A. Mandl, J. E. Manson, M. J. Stampfer and G.C. Curhan (2003), 'Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis', Circulation, 107, 1303-07
Symmons, D. P., C. R. Bankhead and B. J. Harrison (1997), 'Blood transfusion, smoking and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England', Arthritis & Rheumatism, 40, 1955-61
Symmons, D.P.M. and I. Bruce (2006), 'Management of Cardiovascular Risk in rheumatoid arthritis and systemic lupus erythematosus' Reports on the Rheumatic Diseases (published by the Arthritis Research Campaign) 5 (8), 1-4
Tam, L.-S., B. Tomlinson, T.T-W. Chu, M. Li, Y-Y. Leung, L-W. Kwok and T.K. Li (2008), 'Cardiovascular risk profile of patients with psoriatic arthritis compared to controls – the role of inflammation', Rheumatology, 47, 718-23
Van Doornum, S., G. McColl and I. P. Wicks (2002), 'Accelerated Atherosclerosis. An extraarticular feature of rheumatoid arthritis?', Arthritis & Rheumatism, 46 (4), 862-73
van Halm, V.P., M. T. Nurmohamed, J. W. R. Twisk, B.A.C. Dijkmans and A.E. Voskuyl (2006), 'Disease-modifying anti-rheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study', Arthritis Research and Therapy, 8 (5), R151
Voskuyl, A. E. (2006), 'The heart and cardiovascular manifestations in rheumatoid arthritis', Rheumatology, 45, iv 4-iv 7
Walberg-Jonsson, S., H. Johansson, M. L. Ohman and S. Rantapaa-Dahlqvist (1999), 'Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset', Rheumatology, 26, 2552-71
To cite this paper please use the following details: Fraser, O. and Marshall, T. (2010), 'Is Cardiovascular Risk being Effectively Monitored and Managed in Patients with Inflammatory Polyarthritis?', Reinvention: a Journal of Undergraduate Research, Volume 3, Issue 2, http://www.warwick.ac.uk/go/reinventionjournal/issues/volume3issue2/fraser Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk