Investigating the Relative Importance of Parentage versus Environmental Factors on Heterochrony in Basommatophoran Freshwater Snail Radix balthica
Myriam Vanderzwalmen[1], Faculty of Science and Environment, University of Plymouth
Abstract
The importance of genetics in inter-specific heterochrony has been known since the seventies; however, intra-specific heterochrony was believed to be driven by environmental variation (Spicer and Rundle, 2007). Recent studies on Radix balthica showed higher similarity in developmental timing across generations in genetically similar embryos than in distantly related individuals (Tills et al., 2010). In the present study, the relative importance of parentage and egg-mass origin is compared with the environmental importance in generating the heterochrony in Radix balthica. Six developmental events were recorded daily. The analysis of the variations showed the parentage influencing variation in developmental timing to be more important than environmental conditions. Only hatching was affected by both the environment and parentage. When comparing the sequence of developmental events, a strong link was found between sequence variation and nil mortality in the same treatment. The link was found for both treatments. This link was found between parents and between egg-masses from a single parent. This study shows that timing of developmental events is influenced by parentage whereas the sequence of developmental events is influenced by treatment. These findings indicate that both genetics and environmental plasticity influence intra-specific heterochrony but in different ways.
Keywords: Environmental variation, developmental event timing, heritability of environmental adaptation, heterochrony, intra-specific variation, Radix balthica
Introduction
Heterochrony
An evolutionary link between ontogeny (an organism's development) and phylogeny (the evolution of taxonomical groups) was first proposed by Darwin (1859) and De Beer (1930) who both believed that changes occurring during the development of organisms could become important in the evolution of new species and that it could be a mechanism driving speciation. Despite Darwin's and De Beer's ideas of linking ontogeny and phylogeny, evolutionary studies tend to focus on either ontogeny or phylogeny but rarely on both. On the ontogeny level, studies tend to focus on differences observed between individuals of a single species or population. On the phylogeny level, species are studied to try to understand their evolutionary past and investigate what drives differences between the species. Heterochrony is an evolutionary concept linking ontogeny and phylogeny.
Heterochrony is described as the variation in developmental timing between ancestor and descendant. Heterochrony became prominent with Gould and his work 'Ontogeny and phylogeny' (1977). Since the late 1970s the studies researching the link between ontogeny and phylogeny through heterochrony have mostly focused on comparing the development of species belonging to the same taxonomical group (e.g. Spicer et al., 2011; Klingenberg and Spence, 1993; Mabee et al., 2007; McNamara, 1982). In heterochrony existing integrated processes within organisms are shifted in time during an organism's ontogeny (Raft and Kaufman, 1983). Heterochrony during an organism's ontogeny on which selection can act and therefore drive evolutionary change.
Intra-specific vs inter-specific heterochrony
Heterochrony is often studied by comparing the developmental timing and the sequence of developmental events of different species, often species closely related. Overall results show that heterochrony between different species is a result of genetic variation amongst the studied species (Klingenberg and Spence, 1993; Smirthwaite et al., 2007; Slatkin, 1987) with some studies suggesting that the environment can produce phenotypic variation that can become genetically encoded heterochronic change over time (Grünbaum et al., 2007; McCormick,1994; Mensch et al., 2008). The variation in the developmental timing within a single species however has long been believed to be a result of different environments acting on individuals and driving the observed changes (Spicer and Rundle, 2007).
A study by Smirthwaite et al. (2007) on 12 basommatophoran snail species found extensive heterochrony between the species and showed that the observed heterochrony might have partially driven the evolution of the different species in the studied group. Radix balthica, one of the 12 species used by Smirthwaite et al. (2007) has been found to show variation in the developmental timing between individuals within this species (Tills et al., 2010). Recently Tills et al. (2011, 2013) suggested a potential genetic basis for timing differences between individuals of the snail Radix balthica. They found timing differences in the onset of developmental events between embryos of the same population. The genetic association became apparent when they found that individuals with the same time of onset of all developmental events studied had greater genetic similarities than individuals showing variation in the timings of development. This variation could provide the raw material on which selection may act on an intra-specific level, leading to the formation of heterochrony on an inter- specific level, hence linking ontogeny to phylogeny (Tills et al., 2010, 2011, 2013).
Aim
The relative importance of genetics and the environment in generating heterochrony has not been studied previously. The aim of this study was to investigate the extent to which differences in the timing of developmental events and the sequence of developmental events between individuals from the same population are generated by environmental stress, as opposed to being determined by parentage or egg-mass origin between those individuals.
Climate change is accelerating environmental change through a variety of factors such as temperature increase; increase in CO2 levels and for estuarine habitats, an increase in salinity caused by rising sea levels. The ability of species to adapt to rapidly changing environments will affect their survival chances. These environmental changes will also influence ecologically driven evolutionary changes. It is therefore important to understand which mechanisms of evolutionary change the environment drives and which are driven by genetic variation.
We hypothesise that parentage will have a greater influence on the developmental timing of Radix balthica embryos as suggested by Tills et al. (2011, 2013). The physiological response as well as the survival rate of embryos from the various parents could show possible increased adaptations to specific environments that are genetically based.
Materials and methods
General layout of the study
This was investigated by exposing Radix balthica to increased salinity during the embryonic development events: the appearance of eye spots, the appearance of the heart, the timing of foot attachment, the beginning of crawling within the egg capsule, the beginning of radula movement and the timing of hatching (Smirthwaite et al., 2007). The parentage and the egg-mass origin for each embryo were recorded to allow comparisons at the parental and egg-mass level. The effect of increased salinity on the physiology of developing embryos was investigated by measuring the daily heart rate of all the embryos. Comparing the development of the embryos sorted by treatment, parentage and egg-mass allowed the investigation of relative importance of each of those factors.
Radix balthica
The Radix balthica was chosen in this study, as it is an estuarine species known to have extensive variations in its developmental timing within the species that can be affected by salinity (Tills et al., 2010; Jarne and Delay, 1990). Radix balthica was the species used by Tills et al. (2011) to show a link between genetic differences and variation in the timing between individuals of the same population. In addition, it is a species which breeds well in the laboratory and produces egg-masses frequently. It is a hermaphrodite species with asexual reproduction, meaning offspring are genetically more closely related to the parents and siblings than sexually reproducing organisms would be. The embryos are small and transparent. It is therefore easy to observe the embryo's development under a microscope.
Embryo collection and maintenance
Embryos used in this present study originated from a stock population collected from the River Dart in Totnes (Devon, UK 50° 25' 55.2" N, 3° 41' 2.4" W) prior to 2013. The population was maintained for three generations in a 15°C temperature control room. The individuals were exposed to natural daylight and maintained in artificial pond water (APW) (ASTP standard recipe: MgSo4 24.5g; NaHCo3 19.5g; KCl 0.8g for 100L pH=7.4). The individuals were fed a 2cm diameter piece of lettuce every three weeks. Each individual was kept in isolation (vol.= 268ml) and reproduced asexually, mostly lying egg-masses on Elodea pondweed (Tills et al., 2013). A total of 206 embryos were collected from 45 egg-masses originating from 23 different parents. From the original 206 collected embryos from 23 different parents, the data from 90 embryos originating from four parents were retained. The parental origin as well as the egg-mass origin of each embryo was recorded in order to allow analysis of variation to be carried out, knowing the parentage between each embryo.
Egg-masses were collected daily from the F3 generation. Embryos (with an intact egg capsule) for use in the experiment were isolated from egg-masses that had not developed past the shell ridge stage. Embryos were placed individually into wells of a 96 flat bottom multiwell plate (well capacity= 0.4 ml) (manufacture: Sterilin Ltd., UK) immediately after removal from the egg-mass. The embryos were exposed to either a control treatment of APW (see above) or APW with 3ppt of Instant Ocean™ salt (Na+: 462 mmol kg-1; K+: 9.4 mmol kg-1, Mg++: 52 mmol kg-1; Ca++: 9.4 mmol kg-1; Sr+: 0.19 mmol kg-1) (pH= 7.4) throughout their development. The salinity level of the 3ppt treatment was checked daily using a hand refractometer (manufacture: ATAGO, ChemLab Scientific Products Ltd., Japan) (S/MILL) and the pH of water from both treatments was measured using a pH-meter (manufacture: Mettler Toledo FiveEasy) and adjusted, as necessary to maintain constant pH, using 0.1m HCl.
The embryos were placed randomly across the multiwell plate. A single embryo was placed per well, with five embryos of each treatment per plate. This was to avoid any possible 'plate effect'. Water was changed daily to maintain constant salinity and pH levels as described above. The wells were filled to about ¾ to avoid desiccation of embryos due to evaporation. In addition, the multiwell plates were always covered with a lid to further prevent desiccation. Embryos were kept in a 20°C temperature control room with a 12 hours light/dark regimen using 4 Aquaray™ Natural Day Light AO927 11-05-21 CXP-ND full spectrum at 20%.
Development recording
Following Smirthwaite et al. (2007), six morphological developmental stages known to show heterochrony in basommatophoran snails were recorded in the embryos: shell ridge, appearance of eye spots, appearance of the heart, attachment of the foot to the egg capsule/crawling, movement of radula and hatching (Table 1 and Figure 1). Each developmental stage and event was recorded when it was first observed, observations were made every 24 hours.
Observations were done on each embryo daily to record their development using an Optem™ x70 magnitude microscope. A daily photograph was taken of each embryo using an ALLIED-PIKE F210C Vision Technologies™ imaging system mounted on the microscope and an AVT Smartview™ 1.11 software system. A photograph of a measuring scale (manufacturer: Graticules Ltd. Tonbridge, Kent, England UK) (scale: 100x0.1= 10mm) was also taken with embryo photograph to allow standardised measurements of embryo length and width. These measurements were subsequently used to calculate embryo volume. The volume was calculated for each day of development for all embryos following Taylor (1973). The used equation was: 1/6πLW2 (L: length W: width). In addition, the egg volume was calculated following the same method for the day of the shell ridge appearance.
Physiological changes were tracked daily by heart beats per minute starting the day the heart appeared or once the heart rate was regular enough to allow measurement to be taken.
Once hatched, embryos were transferred into glass jars (vol.= 35ml) containing water of the treatment in which they developed, a 5cm long piece of Canadian pondweed, Elodea was added to the jar. Hatched embryos were maintained in the same 20°C temperature control room with 12 hours light/dark regimen as the one used for embryo housing (see above). Four weeks after hatching, an eight mm in diameter piece of lettuce was fed to the hatchlings.
| Event | Description |
|---|---|
| Shell ridge formation | Start of distinction between shell and developing foot |
| Eye spot appearance | Formation of eye spots with darkened pigmentation |
| Heart appearance | Movement of heart chambers |
| Foot attachment | Foot attaches to egg capsule |
| Crawling | Crawling within the egg capsule |
| Radula movement | Forward motion of radula appendix within the head area |
| Hatching | Rupture of egg capsule and subsequent free crawling of juvenile |
Table 1: Descriptions of the developmental events and stages recorded (identified by Smithwaite et al. (2007) and Tills et al. (2011)).
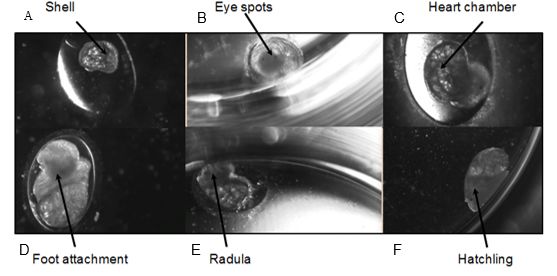
Figure 1: Developmental stages of Radix balthica (identified by Smithwaite et al. (2007) and Tills et al. (2011)): A) appearance of shell ridge; B) appearance of eye spots; C) appearance of heart chambers; D) attachment of the foot onto egg capsule; E) beginning of radula movement; F) hatched embryo.
Statistical analysis
The data was filtered after initial statistical analysis due to high levels of error linked to a high amount of embryos originating from parents which produced a small number of embryos (i.e. average of only one, two or three embryos). Reducing the overall number of embryos increases the robustness of the statistical analysis carried out. Out of the initial 23 parents and the 206-hatched embryos, four parents were selected to have produced enough offspring to be analysed and the data from a total of 90 embryos remained.
The timing of developmental events was recorded from the time the shell ridge appeared and not the collection time. This was done due to the fact that collected embryos were at various stages of gastrulation and the start of development had to be standardised at an identifiable event for correct analysis. For the embryos that survived to hatching, two two-way nested ANOVAs were carried out for each developmental event and daily heart rates using Minitab 16 Statistical Software. The ANOVAs were carried out using the general linear model. Parental origin and water treatment were set variables and egg-mass origin and egg volume was treated as a co-variable. Egg-mass origin and egg volume were set as co-variables to detect any possible effect on the developmental timing below the level of parental origin.
The variation in the sequence of developmental events was compared by plotting on a pantograph the order and the timing of developmental events by parentage and by treatment. Each observed sequence variation for each parent was plotted against the most frequent developmental sequence from the respective parent. This allowed visual comparison of any variation in the sequence of developmental events. Each egg-mass showing variation for parent 1 was also plotted on a pantograph to allow comparisons of the developmental sequence between the egg-masses and the treatment to which the embryos were exposed. Again, each sequence variation was plotted against the most frequent sequence from the respective egg-mass.
Mortality was assessed for the embryos collected from the four selected parents. The mortality was calculated in percentage for the total number of embryos produced by each parent. Mortality had to be calculated this way because each parent produced a different number of embryos therefore calculating the percentage allowed standardisation between the parents. Mortality per treatment was assessed for the egg-masses from parent 1 to allow analysing the effect of increased salinity below the level of parent.
Results
Alterations in the timing of developmental events in embryos that survived
The developmental time of events showed less variation between embryos that originated from the same parents than between those that originated from different parents. However within a parent, the timing of developmental events was different for the two treatments. Increased salinity tended to delay the development to varying degrees between the different events as well as to a varying degree between the parents (Figures 2–7). The majority of events were slowed down by increased salinity, with the exception of the timing of the heart appearance in parent 1 (Figure 3) and the timing of radula movement in parent 2 (Figure 6) where the timing of development in the control occurred later than in the increased salinity. This variation however was minimal compared to the other variations. A Two-way ANOVA using a general linear model was carried out on each developmental event separately to test for the relative effect of parental origin, egg-mass origin and exposed treatment. All four factors, parental origin, egg-mass origin, treatment and egg volume had no significant effect on developmental timing of eye spots (n= 76 F= 0.15 p= 0.927, F= 0.01 p= 0.926 and F= 0.89 p= 0.350, F= 0.07 p= 0.791 respectively R2= 2.73%). The timing of the appearance of the heart was dependent on the parental and egg-mass origins (n= 76 F=2.90 p= 0.042 and F= 8.34 p = 0.005 respectively R2= 19.44%). The treatment and egg volume had no significant effect on the timing of the heart appearance (n= 76 F= 0.53 p= 0.470, F= 1.74 p= 0.191 respectively).
The timing of foot attachment and the start of crawling were observed simultaneously except for one instance and the results of the statistical analyses are therefore reported together. The parental origin was found to have a significant effect on the timing of foot attachment and crawling (n= 76 F= 3.80 and F= 3.88 respectively p < 0.02 for both events R2= 18.89%). However the egg-mass origin, the treatment and the egg volume had no significant effect on the timing of foot attachment and crawling (n= 76 F= 0.51 p > 0.4 and F= 1.85 p > 0.1, F= 0.51 p> 0.4 respectively). The timing of radula movement was significantly varying when analysing the parental origin (n= 76 F= 2.75 p = 0.05 R2= 14.81%) however egg-mass origin, the treatment and egg volume had no significant effect on developmental timing (n= 76 F= 1.45 p > 0.2 and F= 0.40 p > 0.5, F= 0.45 p= 0.504 respectively). The timing of hatching was the event found to have the most variation. Both parental origin and treatment were found to significantly affect the timing of hatching (n= 76 F= 6.90 p > 0.001 and F= 6.29 p > 0.01 respectively R2= 35.14%). The egg-mass and the egg volume had no significant effect on the timing of hatching (n=76 F= 0.11 p =0.739 and F= 1.37 p = 0.246 respectively).
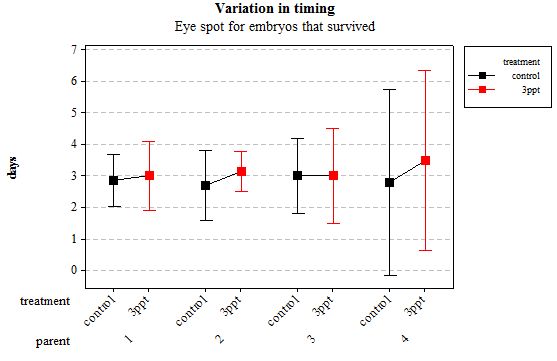
Figure 2: Mean (±95% CI) variation in timing of eye spot appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
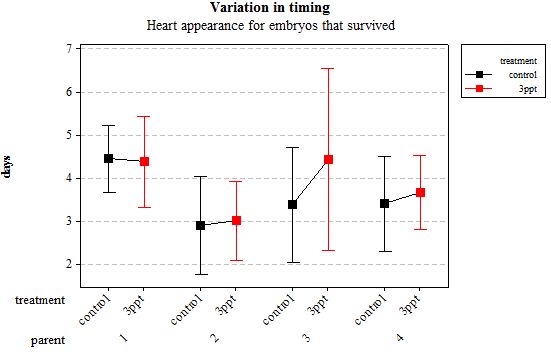
Figure 3: Mean (±95% CI) variation in timing of heart appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
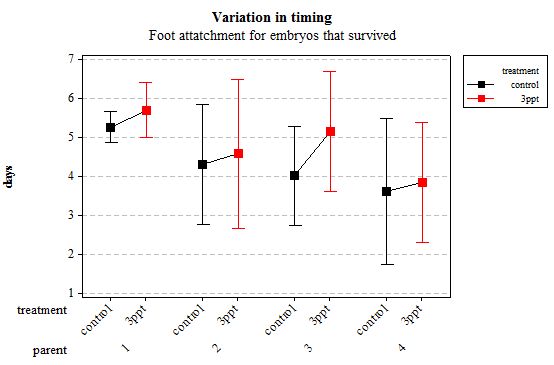
Figure 4: Mean (±95% CI) variation in timing of foot attachment in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
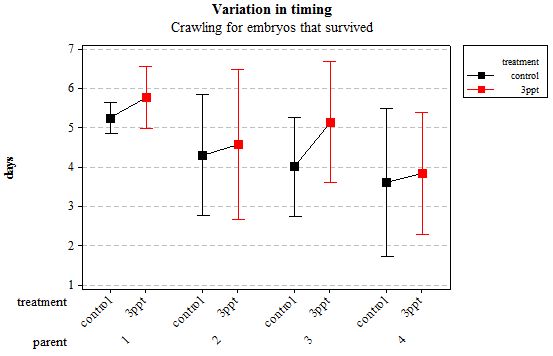
Figure 5: Mean (±95% CI) variation in timing of crawling in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
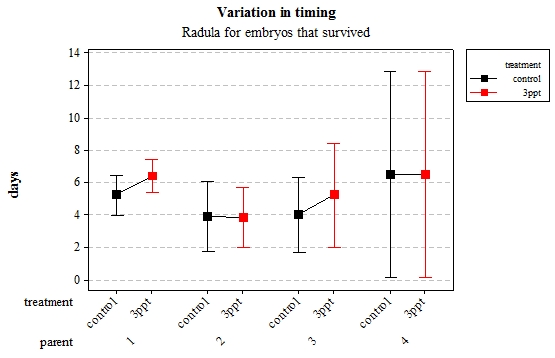
Figure 6: Mean (±95% CI) variation in timing of radula movement in R. balthica by parentage and treatment (control and 3ppt) for embryo
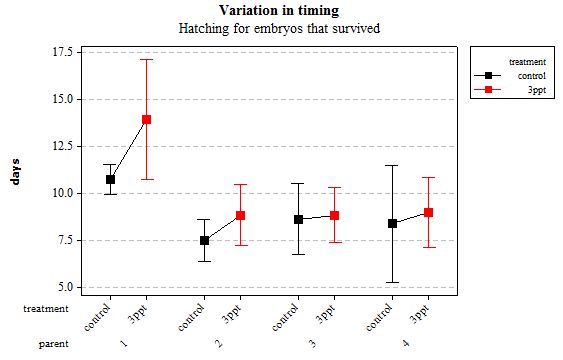
Figure 7: Mean (±95% CI) variation in timing of hatching in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Alterations in the timing of developmental events in embryos that died
The same analyses were carried out for the embryos that died during their development. Analysing embryos that died during their development did not show a clear trend in the developmental timing either. Instead increased salinity both slowed down and accelerated the timing of certain events with no clear pattern found (Figures 8– 12). For all of the events, the only significant effects obtained were by the egg-mass origin on the timing of foot attachment and crawling (n= 16 F= 2.00 and F= 18.00 respectively p < 0.02 for both events). Parental origin was also found to have a significant effect on the timing of foot attachment and crawling in embryos (n= 16 F= 55.37 and F= 56.47 respectively p < 0.02 for both events).
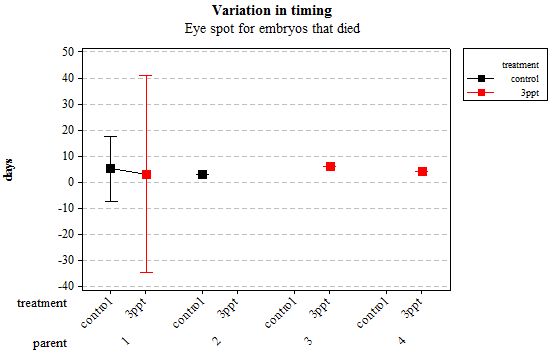
Figure 8: Mean (±95% CI) variation in timing of eye spot appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
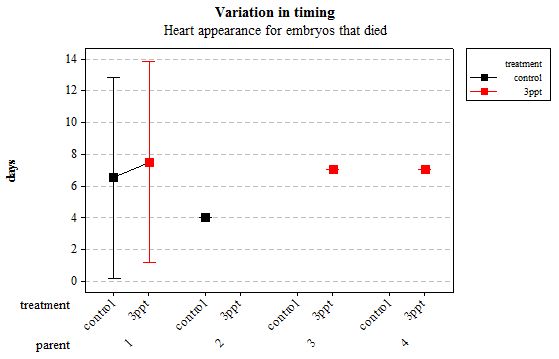
Figure 9: Mean (±95% CI) variation in timing of heart appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
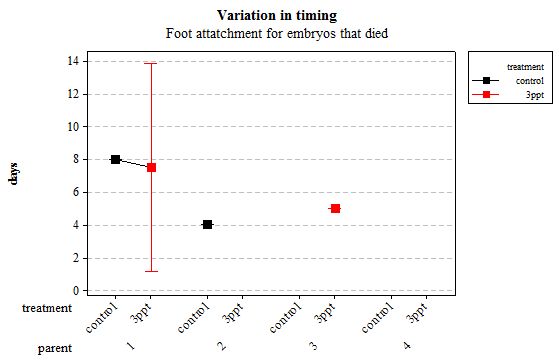
Figure 10: Mean (±95% CI) variation in timing of foot attachment in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
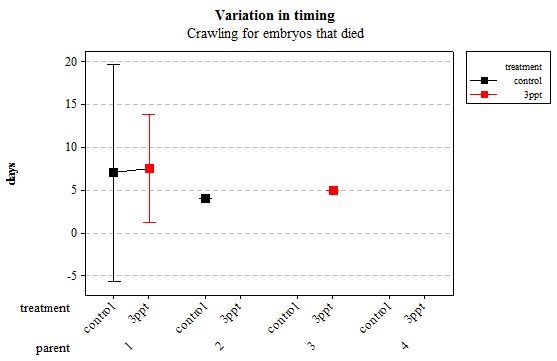
Figure 11: Mean (±95% CI) variation in timing of crawling in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
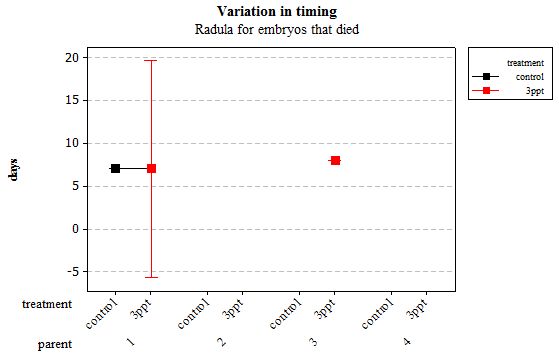
Figure 12: Mean (±95% CI) variation in timing of radula movement in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Alteration in the sequence of developmental timing
The comparisons of the sequence of developmental events plotted on a pantograph showed that the embryos of parent 1 and parent 4 had undergone variation in the sequence of development in both the control APW treatment and the 3ppt salinity APW treatment. The embryos of parent 2 and parent 3, however, showed variation in sequence of development in only one treatment (3ppt salinity APW for parent 2 and control APW for parent 3) (Figure 13). There was no consistency found in the variation of the order of developmental events within either treatment.
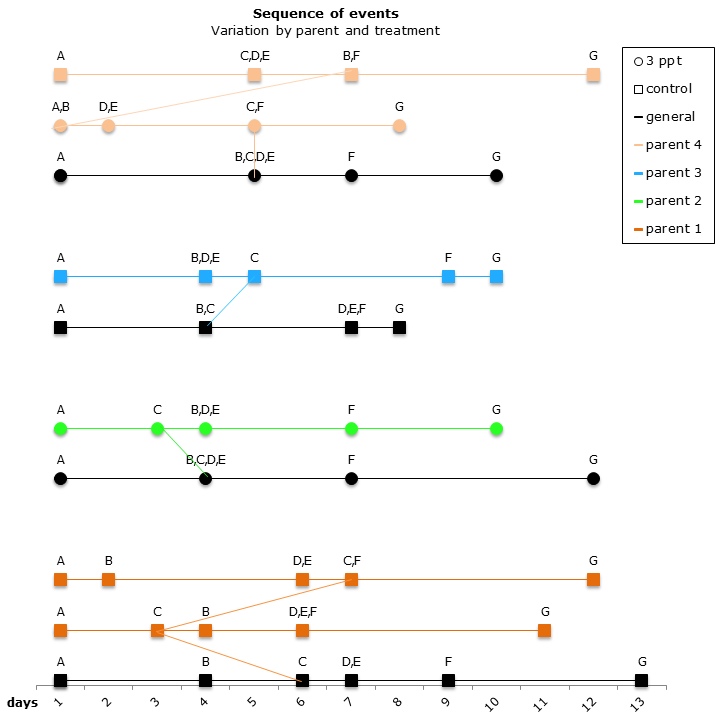
Figure 13: Changes in the sequence of developmental events R. balthica by parent and treatment. A) appearance of shell ridge B) appearance of eye spots C) appearance of heart chambers D) attachment of the foot onto egg capsule E) beginning of radula movement F) movement of radula G) hatched embryo.
The comparisons of the sequence of developmental events within a parent that showed variation in both the control APW and the 3ppt salinity APW, was done by comparing different egg-masses from parent 1. Embryos in five egg-masses out of nine from parent 1 showed variation in the sequence of developmental events. Three egg-masses showed variation only in the control APW and two showed variation in both the control APW and the 3ppt salinity APW (Figure 14). There was no consistency found in the variation of the order within either treatment, thus similar to what was found when comparing the sequence between parents.
Heart rate
Daily heart rates for the selected parents were analysed using two-way nested ANOVA using general linear model. Neither the treatment nor the origin of parent/egg-mass was found to have a significant influence on the daily heart rate of the embryos (Figures 15 and 16).
Mortality
From the original 206 collected embryos from 23 different parents, 106 embryos survived and 26 died before they hatched. Seventy-four embryos were lost during regular maintenance; the data from 90 embryos were retained originating from four parents. Of those 90 embryos, 76 embryos hatched and 14 died during their development. Out of the remaining 14 embryos from the filtered data, eight embryos died for the control APW treatment and six died for the 3ppt APW treatment. The mortality data was assessed for each treatment and parent of the filtered data. Parent 1 and parent 4 had a mortality rate between 15–35% in both treatments. Parents 2 and 3 however had mortality in only one treatment with nil mortality in the other treatment (Figure 17). Mortality per treatment was assessed for the egg-masses from parent 1. Apart from the egg-masses with nil mortality, all egg-masses showed mortality in the 3ppt salinity APW treatment. Three egg-masses showed mortality in the control APW as well as in the 3ppt salinity APW (Figure 18).
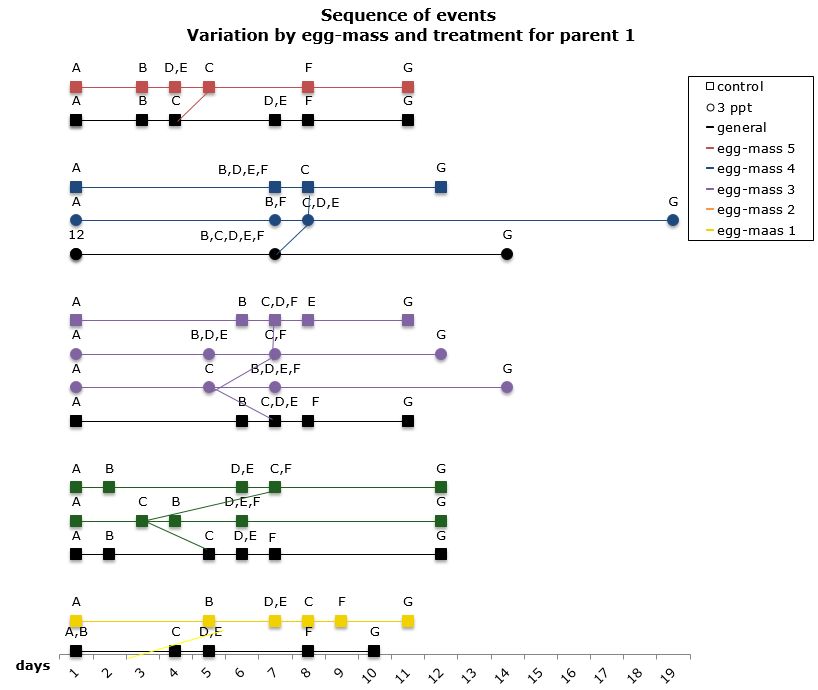
Figure 14: Changes in the sequence of developmental events R. balthica by egg-mass and treatment for parent 1. A) appearance of shell ridge B) appearance of eye spots C) appearance of heart chambers D) attachment of the foot onto egg capsule C) beginning of radula movement F) movement of radula G) hatched embryo.
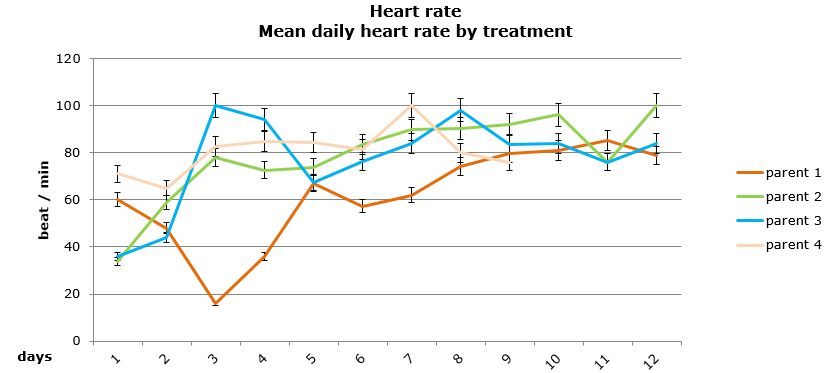
Figure 15: Mean daily (±95% CI) heart rate (beat/min) for embryos that survived by parental origin.
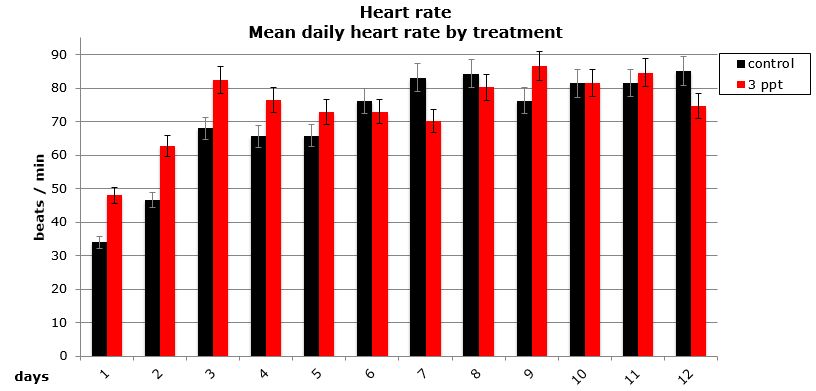
Figure 16: Mean (±95% CI) daily heart rate (beat/min) for embryos that survived by treatment (control and 3ppt).
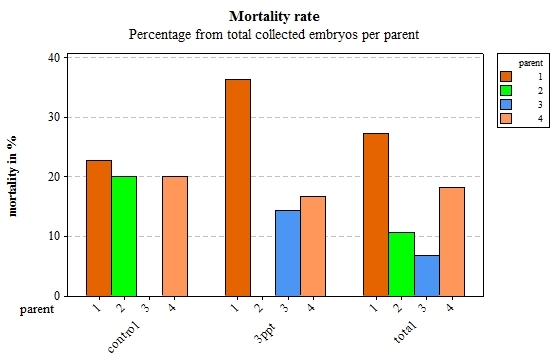
Figure 17: Percentage of embryos that died per parent by treatment (control and 3ppt) and for the total of collected embryos for each parent.
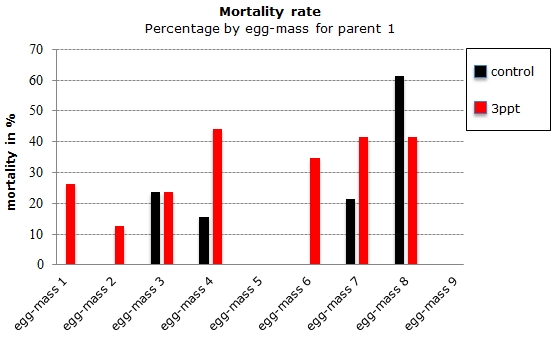
Figure 18: Percentage of embryos that died during the development per egg-mass by treatment (control and 3ppt) for parent 1.
Discussion
In this study we investigated the relative importance of genetic similarity by parentage and egg-mass origin versus the importance of environmental variation on intra-specific heterochrony in Radix balthica. The only data obtained from embryos that survived post hatching was treated to avoid diminishing possible observed effects.
The variation in the sequence of developmental events appeared to be a response to the environment to which the embryo is exposed. Variation in different parents and egg-masses was found in the control treatment alone (parent 3; egg-mass 1, 2 and 5); in the increased salinity alone (parent 2) and in both treatments together (parent 1 and 4; egg-mass 3 and 4). This suggests some parental adaptation to a specific environment.
Mortality rates varied from 0% to 35% in the increased salinity treatment and from 0% to 20% in the control treatment. This shows great variability in the mortality level between environments. Embryos that originated from parent 1 and parent 4 showed relatively similar mortality levels in both treatments whereas embryos that originated from parent 2 showed high mortality levels in the control treatment and no mortality in the increased salinity treatment. Mortality levels in embryos from parent 3 however were the opposite of those in parent 2, no mortality in the control treatment and elevated mortality in the increased salinity environment. Mortality within parent 1 showed that overall embryos have a higher mortality rate in the increased salinity treatment than they do in the control treatment.
When comparing the level of mortality to the variation in sequence a strong correlation was found at both parent and egg-mass level. The parents and egg-masses showing variation in the sequence of development in a particular treatment showed nil mortality in that same treatment. The parents and egg-masses showing variation in the sequence of development in both treatments showed mortality in both treatments as well. This is strong evidence that the variation in the sequence of developmental events undergone by embryos in certain treatments allows them to better adjust to the treatment and therefore lowers the mortality observed in that particular treatment. The variation in the sequence of developmental events appears to be a plastic response depending on the environment to which the embryo is exposed.
The embryos that survived post hatching showed no significant variation early in their ontogeny. These findings correspond to the findings by Smirthwaite et al. (2007) who found variation in the development from mid to late ontogeny. All five developmental events recorded after the appearance of eye spots showed significant variation in the timing of embryonic development depending on parental origin. The results from this study showed that the intra-individual variation in the developmental timing of R. balthica embryos is mostly influenced by the parental origin. The observations support the findings by Tills et al. (2011, 2013) who found parent-offspring similarities in the developmental timing. The results showed that the environment had no effect on the timing of development in R. balthica. Hatching was the only event found to be influenced by both the parental origin and the treatment to which the embryos were exposed, indicating the timing of hatching is influenced by both a genetic component and environmental plasticity. The significance of egg-mass origin in the appearance of the heart suggests a potential similarity in the development between embryos that originated from the same egg-mass in the early developmental events that show heterochrony in the basommatophoran group. However this potential influence needs further investigating with larger egg-masses than were used in this study as the small size of some egg-masses in this study may have hidden the full extent to which egg-mass has an effect on the development of R. balthica.
Foot attachment and crawling were the two events previously found to show heritability (Tills et al., 2011). These two events were found to be influenced by egg-mass origin and parentage; these findings are similar to the findings by Tills et al. (2011).
Conclusion
Tills et al. (2011) showed that heterochrony between individuals of a same species driven from both genetic difference and environmental conditions. However, the way in which both of these factors drive heterochrony was uncertain This study has shown that the influence of the genetics and the plasticity on the developmental variation in Radix balthica is different. The genetic component of the variation was found to affect only the timing of the developmental events. Except for the timing of hatching, only the genetics were found to affect the timing of the developmental events. On the other hand, only the environment was found to influence the sequence of developmental events and only the timing of hatching. It is unclear from this study to what level the genetics or the environment influences the timing of hatching. Further investigations including genetic analyses and continuous monitoring of developmental events starting at the first cell division could show the full extent of the links found in this study.
Multiple studies are needed to understand to what extent the findings of this study apply to other taxa and how the interaction of multiple environmental factors is important in driving intra-specific heterochrony compared to genetics. Carrying out a similar study between closely related species could provide the missing link between ontogeny and phylogeny if both the genetics and environmental plasticity are found to drive inter-specific heterochrony in the same way that they were found to drive intra-specific heterochrony in this study.
Acknowledgements
I would like to thank John Spicer for his guidance and advice throughout the process of this research project. Many thanks to Dr Oliver Tills for giving me access to his snail population, his equipment and for all of the helpful advice he gave during this study. I would also like to thank Manuela Truebano Garcia for allowing me out-of-hours access to the laboratory.
List of Figures
Figure 1: Developmental stages of Radix balthica (identified by Smithwaite et al. (2007) and Tills et al. (2011)). A) appearance of shell ridge B) appearance of eye spots C) appearance of heart chambers D) attachment of the foot onto egg capsule E) beginning of radula movement F) hatched embryo.
Figure 2: Mean (±95% CI) variation in timing of eye spot appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Figure 3: Mean (±95% CI) variation in timing of heart appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Figure 4: Mean (±95% CI) variation in timing of foot attachment in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Figure 5: Mean (±95% CI) variation in timing of crawling in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Figure 6: Mean (±95% CI) variation in timing of radula movement in R. balthica by parentage and treatment (control and 3ppt) for embryo.
Figure 7: Mean (±95% CI) variation in timing of hatching in R. balthica by parentage and treatment (control and 3ppt) for embryos that survived post hatching.
Figure 8: Mean (±95% CI) variation in timing of eye spot appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Figure 9: Mean (±95% CI) variation in timing of heart appearance in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Figure 10: Mean (±95% CI) variation in timing of foot attachment in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Figure 11: Mean (±95% CI) variation in timing of crawling in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Figure 12: Mean (±95% CI) variation in timing of radula movement in R. balthica by parentage and treatment (control and 3ppt) for embryos that died during development.
Figure 13: Changes in the sequence of developmental events R. balthica by parentage and treatment. A) appearance of shell ridge B) appearance of eye spots C) appearance of heart chambers D) attachment of the foot onto egg capsule E) beginning of radula movement F) movement of radula G) hatched embryo.
Figure 14: Changes in the sequence of developmental events R. balthica by egg-mass and treatment for parent 1. A) appearance of shell ridge B) appearance of eye spots C) appearance of heart chambers D) attachment of the foot onto egg capsule E) beginning of radula movement F) movement of radula G) hatched embryo.
Figure 15: Mean daily (±95% CI) heart rate (beat/min) for embryos that survived by parental origin.
Figure 16: Mean (±95% CI) daily heart rate (beat/min) for embryos that survived by treatment (control and 3ppt).
Figure 17: Percentage of embryos that died per parent by treatment (control and 3ppt) and for the total of collected embryos for each parent.
Figure 18: Percentage of embryos that died during the development per egg-mass by treatment (control and 3ppt) for parent 1.
List of Tables
Table 1: Descriptions of the developmental events and stages recorded (identified by Smithwaite et al. (2007) and Tills et al. (2011)).
Notes
[1] Myriam Vanderzwalmen completed her Bsc in Marine Biology in Plymouth in 2013. Over the 2013-14 academic year, she took part in several internships in South Africa and Peru and is currently at the University of Glasgow doing an Msc in Animal Welfare Science Ethics and Law.
References
Darwin, C. (1859), On the Origin of Species, London: Murray
De Beer, G. R. (1930), Embryology and Evolution, Oxford: Clarendon Press
Gould, S. J. (1977), Ontogeny and Phylogeny, Belknap Press
Grünbaum, T., R. Cloutier, P. M. Mabee and N. R. Le François (2007), 'Early developmental plasticity and integrative responses in arctic charr (Salvelinus alpinus): effects of water velocity on body size and shape', Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 308 (4), 396–408, available at http://www.ncbi.nlm.nih.gov/pubmed/17358017, accessed 9 October 2014
Jarne, P. and B. Delay (1990), 'Inbreeding depression and self-fertilization in Lymnaea peregra (Gastropoda: Pulmonata)', Heredity, 64 (2), 169–75, available at http://www.nature.com/hdy/journal/v64/n2/abs/hdy199021a.html, accessed 9 October 2014
Klingenberg, C. P. and J. R. Spence (1993), 'Heterochrony and allometry: lessons from the water strider genus Limnoporus', Evolution, 47 (6), 1834–53, available at http://www.jstor.org/discover/10.2307/2410225?uid=3737592&uid=2129&uid=2&uid=%2070&uid=4&sid=21102396559637, accessed 9 October 2014
Mabee, P. M., K. L. Olmstead and C. C. Cubbage (2007), 'An experimental study of intra-specific variation, developmental timing, and heterochrony in fishes', Evolution, 54 (6), 2091–106, available at http://www.ncbi.nlm.nih.gov/pubmed/11209785, accessed 9 October 2014
McCormick, S. D. (1994), 'Ontogeny and evolution of salinity tolerance in anadromous salmonids: hormones and heterochrony', Estuaries and Coasts, 17 (1), 26–33 available at http://www.jstor.org/discover/10.2307/1352332?uid=2&uid=4&sid=21104263258691, accessed 9 October 2014
McNamara, K. J. (1982), 'Heterochrony and phylogenetic trends', Paleobiology, 8 (2), 130–42, available at http://www.jstor.org/discover/10.2307/2400449?uid=2&uid=4&sid=21104263258691, accessed 9 October 2014
Mensch, J., N. Lavagnino, V. P. Carreira, A. Massaldi, E. Hasson and J. J. Fanara (2008), 'Identifying candidate genes affecting developmental time in Drosophila melanogaster: pervasive pleiotropy and gene-by-environment interaction', BMC Developmental Biology, 8 (1), 78, available at http://www.biomedcentral.com/1471-213X/8/78/, accessed 9 October 2014
Raft, R. A. and T. C. Kaufman (1983), Embryos, Genes and Evolution: The Developmental-Genetic Basis of Evolutionary Change, New York: Macmillan
Slatkin, M. (1987), 'Quantitative genetics of heterochrony', Evolution, 4 (4), 799– 811, available at http://www.jstor.org/discover/10.2307/2408889?uid=2&uid=4&sid=21104263258691, accessed 9 October 2014
Smirthwaite, J. J., S. D. Rundle, O. R. Bininda-Emonds and J. I. Spicer (2007), 'An integrative approach identifies developmental sequence heterochronies in freshwater basommatophoran snails', Evolution & Development, 9 (2), 122–30, http://www.ncbi.nlm.nih.gov/pubmed/17371395, accessed 9 October 2014
Spicer, J. I. and S. D. Rundle (2007), 'Plasticity in the timing of physiological development: Physiological heterokairy – What is it, how frequent is it, and does it matter?', Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology, 148 (4), 712–19, available at http://www.ncbi.nlm.nih.gov/pubmed/17632024, accessed 9 October 2014
Spicer, J. I., S. D. Rundle and O. Tills (2011), 'Studying the altered timing of physiological events during development: It's about time ... or is it?', Respiratory Physiology & Neurobiology, 178 (1), 3–12, available at http://www.ncbi.nlm.nih.gov/pubmed/21699997, accessed 9 October 2014
Taylor, H. H. (1973), 'The ionic and water relations of embryos of Lymnaea stagnalis, a freshwater pulmonate mollusc', Journal of Experimental Biology, 69,143-72
Tills, O., J. I. Spicer and S. D. Rundle (2010), 'Salinity-induced heterokairy in an upper-estuarine population of the snail Radix balthica (Mollusca: Pulmonata)', Aquatic Biology, 9 (1), 95–105, available at http://www.int-res.com/articles/ab_oa/b009p095.pdf, accessed 9 October 2014
Tills, O., S. D. Rundle, M. Salinger, T. Haun, M. Pfenninger and J. I. Spicer (2011), 'A genetic basis for intra-specific differences in developmental timing?', Evolution & Development, 13 (6), 542–48, available at http://www.ncbi.nlm.nih.gov/pubmed/23016938, accessed 9 October 2014
Tills, O., S. D. Rundle, and J. I. Spicer(2013), 'Parent-offspring similarity in the timing of developmental events: an origin of heterochrony?', Proceedings of the Royal Society B: Biological Sciences, 280 (1769), 20131479
To cite this paper please use the following details: Vanderzwalmen, M. (2014), 'Investigating the Relative Importance of Parentage versus Environmental Factors on Heterochrony in Basommatophoran Freshwater Snail Radix balthica', Reinvention: an International Journal of Undergraduate Research, BCUR 2014 Special Issue, http://www.warwick.ac.uk/reinventionjournal/archive/bcur2014specialissue/vanderzwalmen/. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal at warwick dot ac dot uk.