Gene Editing: The Road to Transhumanism?
Hannah Rose Hume[1], Faculty of Biological Sciences, University of Leeds
Abstract
Transhumanism is a movement with the aim of transforming the human condition through technology. Transhumanists believe that humans have the capability to evolve into 'post-humans': that is, both mental and physical capabilities could be improved upon in almost any aspect should the appropriate technology be applied. Gene editing is among these transhumanist technologies, made possible by currently used tools such as TALENs and CRISPR/Cas9, which will be discussed in detail. When it comes to editing the human genome, the possibilities are beyond our current comprehension, but the technology exists to be able to make some of these changes today. In the future, technologies such as CRISPR/Cas9 have the potential to eliminate disease, increase mental skills and increase physical power. Of course, regardless of the technical hurdles faced in researching and implementing these kinds of changes, it brings with it a whole host of ethical, moral and practical issues. Should we make use of this technology at all? If so what precautions should be taken and what should we use it for?
Keywords: Gene editing, Transhumanism, CRISPR/Cas9, TALENs, Designer babies, Germline
Introduction
The Oxford Dictionary definition of transhumanism is 'The belief or theory that the human race can evolve beyond its current physical and mental limitations, especially by means of science and technology' (Oxford University Press, 2017). There are many means by which the transhumanist movement believes this can be implemented, from bionic limbs (Honigsbaum, 2013) to more futuristic technologies such as uploading the mind to a computer (Bartlett, 2014; Bostrom, 2003b) and cryonics. Many aims of transhumanism such as eradication of disease, lengthening the human lifespan and healthspan and increasing physical and mental capacity (Bostrom, 2003b) are desirable and sought after by other areas of research. It is perhaps not these goals that make the transhumanist movement controversial, but the drastic measures through which they aim to achieve them which may potentially change humankind as we know it.
One of these transhumanist technologies, and the focus of this article, is gene editing. While it has proven controversial over the years, it has been well studied in human adult cells and animal embryos and was approved for use in human embryos in the UK in 2015 (although, other than in China and in some US states, it remains widely illegal). Controversially, in early 2015, a group in China became the first to edit human embryos using CRISPR/Cas9 (Clustered Regularly Interspaced Repeats/CRISPR-associated nuclease 9) using non-viable embryos to study the gene responsible for β-thalassemia (Cyranoski and Reardon, 2015). Gene editing in adult human cells is already well understood and is being used in clinical trials for HIV and cancer, with many others on the way. Somatic gene therapy is in clinical use for several disorders including muscular dystrophies, sickle cell anaemia, leukaemia and perhaps most notably severe combined immunodeficiency (SCID). While these therapies are now producing positive results, this wasn't always the case. The first gene therapy trials in the 1990s for SCID proved a disaster for the field of gene therapy when multiple patients developed leukaemia. Ultimately this setback was only temporary and now the risks involved in somatic gene therapy are minimal due to our increased knowledge; however, it highlights the risks involved in interfering with the genome and the risks with germline gene therapy may remain unknown until it is trialled in humans.
Genetic engineering - the general term for manipulating the genetics of an organism, often using recombinant DNA - has been around since the early 1970s and is not to be confused with the much newer technology of gene editing, in which specific changes down to a single nucleotide can be made via engineered nucleases. Among the most promising current technologies are TALENs (Transcription Activator-Like Effector Nucleases) and CRISPR/Cas9. The latter is the most recently discovered and regarded as the simplest, cheapest and most accessible technology, even being named Science magazine's 'Breakthrough of the Year 2015' (Sanders, 2015). Research using CRISPR has soared in the last couple of years at a rate that has people worried about its unknown implications, not least in its potential to edit the human germline (Ledford, 2015). In December 2015, scientists, ethicists, legal experts and members of the public met at an international summit in Washington D.C. to discuss the dangers and ethics of editing the human genome (ONPI, 2015). The need for this discussion was met with urgency, and showcased widely divided opinion (Travis, 2015).
The difference between somatic and germline genetic modifications is small in terms of the technology and techniques required, but is vast in terms of ethics. Somatic therapy makes changes to individual adult cells, and affects only the individual receiving treatment. Germline therapy makes changes to an embryo, meaning that the changes made will be present in every cell in the child resulting from that embryo, as well as all future offspring derived from the individual.
Achieving some of the aims of the transhumanist movement is becoming increasingly possible, and this article will focus on one of the technologies allowing this - human gene editing - and will review the science and ethics surrounding it. A background of currently used methods of gene editing will be given, as a brief understanding of these (in particular CRISPR) is important for understanding the magnitude of the technology, the ease with which it could be used to edit the human germline and the pace at which it is moving forward. This is followed by a review of current research and clinical applications, some of the existing ethical debates around human gene editing and the way they are being handled. The literature selected is a mixture of original research papers and reviews on the different technologies, clinical research papers, pieces from relevant websites such as the Transhumanist Party and the National Academies, news articles and sociological opinion pieces. This wide range of literature is necessary to effectively cover all the angles of scientific explanations, clinical applications and ethical implications. As the paper discusses such a fast-moving technology, much of the literature included is from around the original time of writing of the paper.
Genome editing technologies
ZFNs (Zinc-Finger Nucleases) and TALENs
The first site-specific gene editing technology came about in 1996 (Nemudryi et al., 2014) in the form of ZFNs (see Figure 1). Zinc-finger proteins (ZFPs), coupled with a restriction enzyme nuclease domain, can target and bind long recognition sites and allow specific cleavage at a target site chosen by the researcher (Urnov et al., 2010) and subsequent repair by non-homologous end-joining (NHEJ) or homology directed repair (HDR) (see Figure 3).
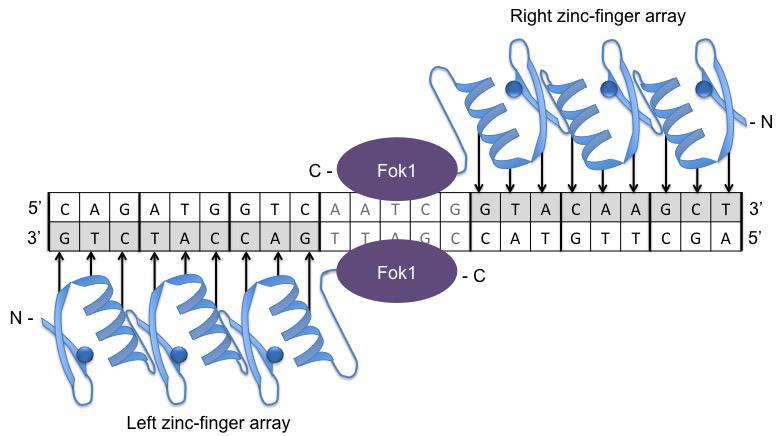
Figure 1: Schematic of a zinc-finger nuclease dimer bound to target DNA. Zinc-finger proteins (blue) bind to a specific DNA sequence (of 3 base pairs per ZFP) either side of the target site allowing the Fok1 restriction enzyme to make a cut at a specific locus.
ZFNs have been used for several years in research in many cell types and organisms and may even have a therapeutic role in treating diseases such as HIV (Perez et al., 2008). However, ZFNs have many drawbacks: they are expensive, complex and time-consuming to assemble as they comprise proteins - the genes must be synthesised and expressed and the products purified, which may take months (Gupta and Musunuru, 2014). Additionally, accurate cleavage of target DNA is not guaranteed (Nemudryi et al., 2014). For these reasons, alternatives have been developed and TALENs are based on similar principles.
TALEs (transcription activator-like effectors) are naturally occurring proteins (Nemudryi et al., 2014) that bind individual bases and, alongside an endonuclease, make up a TALEN (Joung and Sander, 2013). TALENs allow greater flexibility than ZFNs due to recognition of individual bases rather than of groups of three bases, and in theory allow the double-strand break to be introduced at any point in the genome (Gaj, Gersbach and Barbas, 2013), which is then repaired by HDR or NHEJ (see Figure 3).
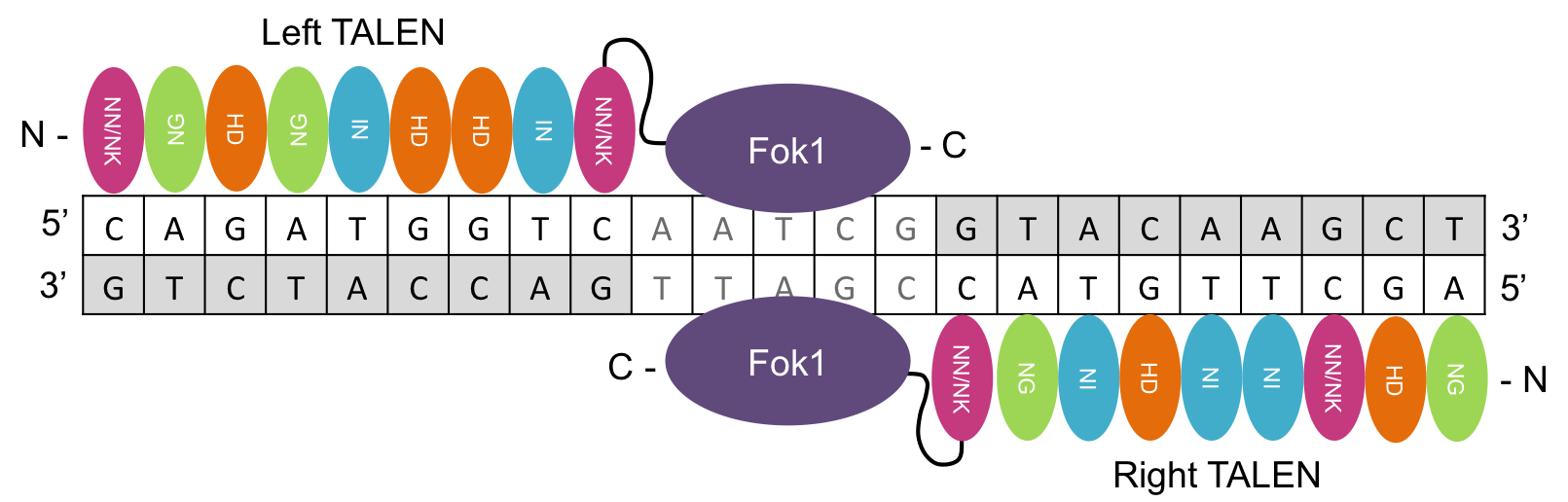
Figure 2: Schematic of TALEN dimer bound to DNA. Each TALE (pink, green, orange, blue) binds to specific DNA base pair, so that TALENs bind either side of the target site allowing Fok1 restriction enzyme to make a cut at a specific locus.
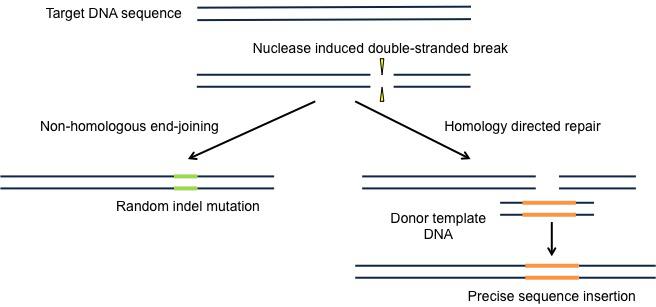
Figure 3: Comparison of repair of nuclease-induced double strand break by NHEJ and HDR. NHEJ can result in relatively imprecise deletions or insertions of variable lengths. HDR can result in precise point mutations, deletions or insertions from a single or double stranded template.
TALENs are simpler, more accurate and less costly than ZFNs and, although the use of both is decreasing to make way for CRISPR/Cas9 (see Figure 5), TALENs still have a place - for example TALENs were recently used to successfully treat a one-year-old girl in London with advanced leukaemia (Maude et al., 2014).
CRISPR/Cas9
CRISPRs - or what would later become to be known as such - were first observed in the genomes of E. coli in 1987 (Ishino et al., 1987). However, their purpose and function remained a mystery until 2007. Then, in June 2012, Emmanuelle Charpentier and Jennifer Doudna's group published a ground-breaking paper demonstrating the potential of CRISPR/Cas9 technology showing that fusing the crRNA and the tracrRNA to create a single molecule capable of guiding the Cas9 endonuclease could create double-strand breaks at a specific site in target DNA (Jinek et al., 2012). It was with this (and other research published around the same time) that CRISPR/Cas9 technology was born.
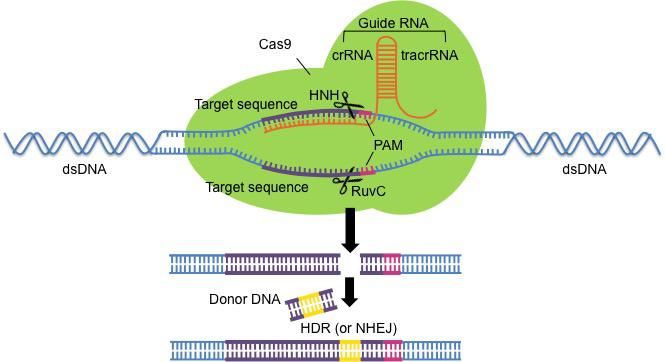
Figure 4: Schematic of gene editing using CRISPR/Cas9. Guide RNA (orange) guides the Cas9 endonuclease (green) to the DNA target sequence (purple). The double strand break made by HNH and RuvC domains can be repaired by NHEJ, or more commonly HDR as long as donor DNA is supplied.
The classic CRISPR/Cas9 system commonly used in the lab comprises wild-type Cas9 alongside a single, synthetic guide RNA made up of a tracrRNA and crRNA fusion. Cas9 cleaves double-stranded DNA at a specific site determined by the design of the guide RNA, causing DSB repair to be initiated either via NHEJ or HDR (see Figures 3 and 5). NHEJ disrupts the gene at the target locus causing deletions or insertions, which can be useful in applications such as assessing the contribution of a certain gene. HDR can occur when a homologous donor template is supplied and is inserted during repair to replace the target sequence with a different, desirable sequence (for example, replacing a disease allele with a wild-type allele).
In practice, the whole CRISPR/Cas9 system is simple and flexible compared with other gene editing technologies. It depends on a small RNA as a guide. These are simple enough to make chemically, as opposed to the proteins used in ZFNs and TALENs that require synthesis and expression of the gene and subsequent purification of the protein product and can be costly and time-consuming. Controlling base pairing between target DNA and the engineered RNA in CRISPR/Cas9 is simple in comparison to controlling protein-DNA interactions in ZFNs and TALENs (Sander and Joung, 2014). In addition, the most commonly used form of CRISPR/Cas requires only two components to function: the Cas9 nuclease and a guide RNA. While ZFNs may cost in excess of $5000 to order, CRISPR components can mainly be bought off the shelf with only the RNA fragment requiring specific design and may cost as little as $30 in total. This has allowed gene editing to become far more accessible (Ledford, 2015) to the point that a crowd funded project is now selling bacteria and yeast DIY CRISPR kits to the general public, containing 'everything you need to make precision genome edits at home' (Zayner, 2015).
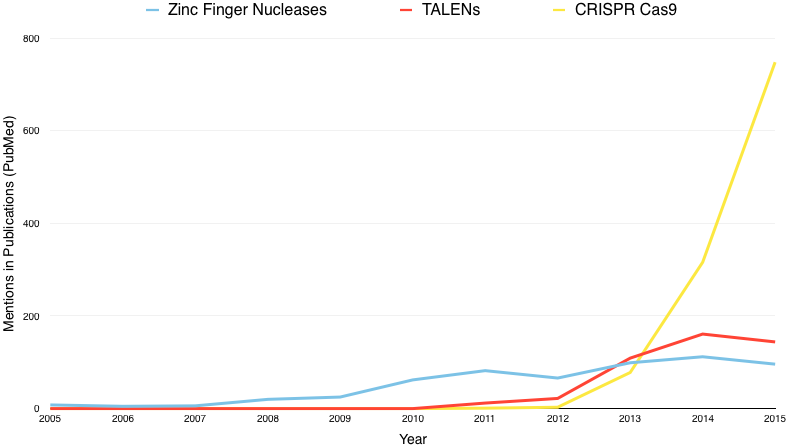
Figure 5: Number of publications on PubMed mentioning ZFNs (blue), TALENs (red) and CRISPR Cas9 (yellow) from 2005 to 2015.
CRISPR is considered one of the biggest breakthroughs in science in recent years, and some already consider it the 'holy grail of medicine'. The number of publications mentioning CRISPR has grown almost exponentially since its discovery (see Figure 4) (Quinlan, 2015), and in the first 51 days of 2016, CRISPR/Cas9 already had 238 mentions across PubMed - an average of 4.66 publications per day. Its quick rise to the forefront means that little time has been devoted to considering the implications that may come from such a powerful tool. In early 2015, Protein and Cell published the first research in which a human embryonic genome had been modified. A Chinese group led by Junjiu Huang modified the gene underlying β-thalassemia (the HBB gene, which encodes human β-globin protein and can cause the blood disorder if mutated) in non-viable human embryos. The embryos were non-viable discards from an IVF clinic and therefore could not have been used to establish a pregnancy, (Hesman Saey, 2015; Liang et al., 2015) but the study caused a major stir as it was unexpected and it showed the potential for designing embryos intended to establish a pregnancy. Huang's group showed that the technology is not ready for clinical use due to off-target mutations and their potential effects, as well as the relatively low rate of successful targeted insertion and uptake of the replacement gene (Cyranoski and Reardon, 2015). Of 86 embryos injected, 71 survived, of which 54 were tested. Only 28 of these showed successful insertion, and very few had taken up the genetic material intended to replace the faulty gene. They also found a greater incidence of off-target mutations in the human embryo than they found in animal embryos and adult human cells and, since they looked only at the exome, this was assumed to be only a small portion of mutations present (Liang et al., 2015).
Following this controversy, the US National Institutes of Health (NIH) reiterated its ban on editing human embryonic genes for research (Liang et al., 2015; Reardon, 2015d). However, a few months later, in the UK, a team at the Francis Crick Institute in London applied for a licence to use CRISPR/Cas9 to edit genes in human embryos for the first time and, in January 2016, it was approved by The Human Fertilisation and Embryology Authority (HFEA) (HFEA, 2016; The Francis Crick Institute, 2016). Unlike Huang's group, the team from London hope only to gain better understanding of the roles of certain genes in development through studying early development of human embryos. While this has faced less controversy, it is the first time a national regulatory body has approved this kind of research anywhere in the world and is therefore a significant step for the technology (Cressey, Abbott and Ledford, 2015).
Ethical issues surrounding transhumanism
Transhumanism is a movement with the aim of transforming the human condition through the use of technology. Although loosely defined, many transhumanists would agree that priorities in achieving this include increasing longevity, intelligence, health and wellbeing. Many technologies already exist with the aim of improving these qualities, and most are not met with the same distrust as gene editing. Technologies such as laser-eye surgery and contact lenses, fertility treatments and contraceptive devices, cosmetic surgery and dentistry are all invasive but commonplace technologies used to improve quality of life. It could be argued that these, too, are (albeit less extreme) transhumanist technologies. Bionic limbs for amputees and robots carrying out tasks once done by humans are becoming more commonplace and, while artificial intelligence or uploading a human memory to a chip may seem like science fiction, they are becoming slowly more feasible without obvious opposition. Gene editing, however, remains highly controversial - in particular germline gene editing to 'design' the next generation of children. Despite this, gene editing is likely to be the most efficient way of achieving many transhumanist goals, and it is no longer the futuristic technology it was merely a few years ago.
Gene therapy and somatic gene editing - the slippery slope to editing the germline
Somatic gene therapy is a well-established area of research, and several treatments are now in clinical trials or on the market. Perhaps the best-publicised example of gene therapy is for treating severe combined immunodeficiency (SCID) (Allenspach, Rawlings and Scharenberg, 2003). Previously, the only treatments were bone marrow transplant, enzyme or immunoglobulin replacement, or living in a sterile plastic 'bubble' - hence the nickname 'bubble-boy disease'. Recent clinical trials have shown success; however, this was not always the case. In 1999, a clinical trial in France successfully cured nine of eleven infants of SCID (Cavazzana-Calvo, 2000). Unfortunately, four out of nine of the successfully treated participants went on to develop leukaemia over the next few years (Hacein-Bey-Abina, 2008; Geddes, 2013). This was thought to be down to insertional mutagenesis caused by the retroviral vector, and this lack of control over the site of insertion in somatic gene therapy makes a case for targeted gene editing.
One example of targeted gene editing in a somatic environment is using ZFNs to treat HIV. This is achieved by extracting blood from patients and using ZFNs to cut out the gene for CCR5, an HIV target protein found on CD4 T cells, before injecting it back into the patient (Tebas et al., 2014). Another is a leukaemia therapy that was recently used successfully for the first time in a one-year-old girl in London (Page, 2016; Reardon, 2015c). T cells are taken from a healthy donor and edited using TALENs - in order to make them exclusively attack the cancer cells in question and not the recipient's cells - before being transplanted into the patient. Within 14 days, the patient was in remission, and remains so to this day. This case was a one-off, last resort treatment, but due to its success, the therapy will be further tested in clinical trials (Qasim et al., 2015).
Prevention of some disorders before birth has been made possible by embryo screening for would-be parents carrying certain genetic diseases, as well as through PGD (preimplantation genetic diagnosis) for couples undergoing IVF. If this could be taken a step further, using gene editing to cut out disease alleles from otherwise healthy embryos and preventing genetic disorders before birth, this may remove the need for other treatments for these diseases. While somatic gene editing would remain relevant for acquired diseases such as cancer and HIV, it still poses the question: should more time and money be put into germline gene editing research than into research for other kinds of treatments?
Germline genome editing: the debate
An international debate on human gene editing was kicked off in December 2015 when the International Summit on Human Gene Editing took place in Washington D.C. (ONPI, 2015). It has been coined as the Asilomar of this generation - the conference in 1975 to discuss (the then new) recombinant DNA technology (Sample, 2015). The Asilomar conference served as a landmark in the history of genetics; however, the 2015 debate has been said to be more important on a wider scale as the concern is not over lab safety, as it was in 1975, but over the potential to change human life as we know it (Berg, 2008).
The 2015 summit was unusual for a scientific event in that it involved not only scientists, but also ethicists, social scientists, legal experts and even victims of genetic disease. Furthermore, it discussed not only recent developments in the science but also issues surrounding ethics, societal problems and how it should be regulated on a small and an international scale (ONPI, 2015). Opinions were varied, unsurprisingly, and ranged from those eager to continue with gene editing research in both adults and embryos (Harris, 2015) to those calling for a moratorium on all further research (Darnovsky, 2015a, b). After three days of discussion, the organising committee came to the conclusions that both basic and preclinical research, as well as somatic clinical use, are providing invaluable knowledge and turning out promising results, and they should be allowed to continue so long as appropriate ethical and legal rules are regarded. It was decided that, at present, germline editing in a clinical setting would be irresponsible while we have inadequate knowledge about safety and ethical implications; however, it did not rule out germline editing in the future and recognised the need to continue to revisit the topic regularly (The National Academies of Sciences, 2015). The consensus was that a moratorium would not only be detrimental in discovering new therapies, but also that it would be unrealistic. CRISPR/Cas9 in particular is already so accessible that to try and impose a ban on it would be near impossible, only serving to encourage unregulated research - in other words, the genie is out of the bottle (Doudna, 2015). While the discussion centred only around human genome editing, CRISPR/Cas9 is used in other areas such as agriculture and gene drive research, and to ban its use would set these back needlessly and harmfully. Instead, the group came to a predictable conclusion: that gene editing in humans should be allowed to continue for research purposes; however, it should not yet be used in embryos that could or will be used to establish a pregnancy. They also affirmed that this issue should be in continuous discussion and that this meeting should be the first of many (Reardon, 2015a, b).
The summit highlighted many cultural and social issues, not least because it took place on an international stage. One major divide in opinion was the question over what can be considered a disorder. Many agree that the prevention of fatal genetic diseases through gene editing should be implemented when it becomes possible, but opinion is more varied over other 'disorders'. For example, people with a mild form of autism spectrum disorder may live similar lives to non-sufferers, and may even have increased creativity or skill in areas like mathematics or music. However, severe autism can prevent normal function in society and standard of life would likely be better if the disorder did not exist. While deafness is considered a debilitating disorder by many, others in the deaf community would not consider themselves disabled (Sharp, 2008) and this difference in opinion has caused controversy on multiple occasions. In 2008, a deaf couple in the UK that had previously given birth to a deaf child through natural means were angered to discover that should they have another child, this time through IVF, that deafness was screened for in the process of PGD and embryos with a gene for deafness would be automatically discarded (Hinsliff, 2008). This demonstrates that what governments and scientists deem to be a disorder may not necessarily match the feelings of those 'sufferers'. Agreeing on rules regarding the categorisation of these disorders at a local level, let alone at an international level, will be challenging - which makes a case for leaving the decision of whether or not to undergo gene editing in the hands of the individual and not governments or scientists.
In order to be truly inclusive, the summit involved members of the public affected by genetic disease. The mother of a baby boy who died six days after birth of anencephaly concluded her speech with 'If you have the skills and the knowledge to eliminate these diseases, then freakin' do it' (Travis, 2015). This highlighted another angle of the debate: the ethical issues in not using the technology for treating disease. While there is concern over the safety of gene editing, bioethicist John Harris points out 'It's not as if the alternative is safe. People with genetic diseases are going to go on reproducing' (Reardon, 2016).
Just because 'the genie is out of the bottle' where CRISPR is concerned, this does not automatically mean that germline gene editing is inevitable. As with all scientific research, whether there is a high enough demand to result in enough funding will ultimately decide if or when germline gene editing takes off. It is certainly not a quick-fix solution for anything and would potentially be viewed as too long-term, costly and uncertain to justify spending money on research grants. However, with large companies such as Google (Calico, 2015) and Microsoft (Microsoft, 2017) currently putting money into other transhumanist technologies such as artificial intelligence and anti-ageing techniques, perhaps there is little reason to believe this might be the case. Either way, the manner in which gene editing moves forward is likely to be determined by the motives of the governments and corporations funding the research. Considerations over social issues may therefore take a backseat to more immediate results ultimately based on profit.
Designer babies and 'playing God'
Controversy around gene editing to treat disease is largely over the slippery slope it may create to improving other traits and making 'designer babies'. This, however, is the whole point of the transhumanist movement. For transhumanists, the option of designing our unborn children to have better memories, be more intelligent, fitter, stronger and to live longer lives is the goal, while for sceptics this is a reason to avoid gene editing altogether.
Social inequality is already rife in almost every society, particularly in developing countries. Suppose genome editing was legalised without restriction, and private companies offered 'baby designing' services for a fee. Only the more affluent - those whose children would already have an advantage in access to education, technology and healthcare - would access these services. If these same children were designed to be more intelligent and in better physical health, for instance, their peers whose parents could not afford enhancements would be at an even greater disadvantage in competition for education, jobs and potential partners. This would all but remove social mobility and widen the class gap further with each generation, with the possibility of evolving into two separate sub-species over many generations (Bostrom, 2004). One way of maintaining a level playing field throughout society would be to make certain enhancements mandatory for all. Of course, if everyone was designed to be taller or more physically attractive, this would no longer confer an advantage. However, if everyone was born in excellent physical health, this would benefit the whole of society as strain on healthcare systems would be reduced. If everyone were to be born with greater mental capabilities, this may not confer an advantage to one individual over another, but it would likely benefit society as a whole. (Bostrom, 2003a). However, while compulsory enhancements for all may solve the class gap issue, it opens up another issue over reproductive freedom. Allowing governments control reproductive rights and/or attempting to improve the gene pool has a chequered past. China's recently abolished one child policy originally introduced in 1979 to slow population growth rate led to the killing of many unwanted children (in particular girls) and has resulted in major problems with an ageing population with over 30 per cent of the population now over 50 years of age (@BBCNews, 2015a). Other examples including forced sterilisation of thousands of underprivileged women in Peru less than 20 years ago in an attempt to drive down birth rates and reduce poverty (@BBCNews, 2015c), and the Nazi regime's 'social cleansing' programme where those with certain genetic conditions were sterilised and certain racial 'alien' groups eliminated (United States Holocaust Memorial Museum, 2015) - both of which clearly demonstrate the dangers of controlled reproduction.
The argument that by interfering with the genome we are 'playing God' may have negative connotations, although it may not necessarily be a bad thing. Humans have evolved to a point where we have no natural predators and are able to treat many would-be-fatal diseases, and so have likely stopped evolving in the way that we did in the past and that other species continue to do (Furness, 2013). While the idea of survival of the fittest works in nature, in human societies the notion that 'the weakest' should not survive long enough to pass on their 'weak' traits to the next generation seems somewhat barbaric - therefore, perhaps we need another form of progression to take the place of evolution. Biologically we are similar to our ancestors from hundreds of thousands of years ago; however, our needs and priorities have changed beyond recognition. Natural selection has only made us good at survival. However, we are not evolutionarily good at important aspects of life such as happiness, for example, as anxiety and aggression used to be key to survival while being too content and relaxed would have been detrimental. The other way around is now true, however we will not see changes through evolution. But, if we could eliminate mental illness and other suffering in a different way, this would greatly increase our wellbeing (Transhumanistparty, 2016b).
Conclusion
Ultimately human life as we know it is constantly changing. In the last decade or so, technology has become increasingly ingrained into our lives and, with the advent of wearable technology such as smart watches and Google Glass, and of course the ever-increasing use of smartphones, we are becoming more reliant on technology every day. There are plans for whole cities to be maintained by robots in the not-too-distant future (University of Leeds, 2015) and people are walking around on prosthetic legs and with medical devices such as pacemakers implanted into their bodies. Diseases that were considered fatal mere decades ago are now treated routinely with commonplace drugs or prevented from ever happening at all through vaccination programmes. It could be said that we are already transhuman - we are living longer and focusing on our wellbeing rather than just survival with technology ingrained into our lives. Technologies with the potential to make big changes are often controversial in their young years, and not all scientific breakthroughs have been met with a warm welcome. When Edward Jenner first suggested infecting someone with cowpox in the hope that it might prevent them from getting smallpox, he was considered crazy. It required a leap of faith - and one that paid off, saving countless lives across the globe through vaccinations against not only smallpox but also a plethora of other fatal diseases. Not many people gave credit to Charles Darwin at the time when he put forward the theory of evolution, and Galileo was sentenced to life imprisonment for his theory of the universe - theories that most current day scientists take more or less as 'fact'. They both took a leap of faith despite opposition and changed science for the better. As for those discoveries that have resulted in disaster, it is unlikely that the scientists who discovered and told the world about nuclear fission wished for it to be used to make nuclear weapons. Equally when electricity, cars and aeroplanes were invented, the scientists involved had no way of knowing that they would cause air pollution, let alone the scale of damage that burning fossil fuels would do to the environment. However, we are living in an age where we are well aware of our past mistakes as a species and are well equipped to learn from them. Even considering the negatives to editing the human genome, should this be a deterrent? In the case of burning fossil fuels for power, although global warming is arguably the greatest challenge the human race currently faces, where would we be as a race without electricity and motorised transport? Most scientific advances of the last century would never have been, and quality of life, life expectancy and scientific knowledge may well have never moved on. When it comes to problems, as a species we will learn to live with and deal with the consequences.
Using CRISPR to edit the human genome could be the scientific breakthrough of a generation, and one that changes life as we know it if it is used to its full potential. At this point, human gene editing and other transhumanist technologies may well be inevitable, and perhaps the only way forward is to embrace this opportunity to better the human race as we have done in so many other instances. In the words of Jennifer Doudna (2015), 'We can now edit our DNA. But let's do it wisely'.
Acknowledgements
I would like to thank my supervisor Dr Kenneth McDowall for his continued support and guidance throughout the project, as well as Dr Dave Lewis and Dr Susan Whittle (all of the Faculty of Biological Sciences, University of Leeds) for helpful discussions and practical advice.
Notes
Hannah Hume graduated from the University of Leeds in 2016 with a BSc in Biological Sciences. As part of her degree she spent a year as a research student in an immunology lab in Osaka, Japan working on projects involving Tregs and other T cell subsets. Since graduating she has worked as a biology teacher in India for five months, worked at a ski resort in the French Alps and is currently travelling before returning home to pursue a career in science.
Figures
Figure 1: ZFN schematic
Figure 2: TALEN schematic
Figure 3: HDR/NHEJ repair comparison schematic
Figure 4: CRISPR/Cas9 schematic
Figure 5: PubMed mentions of ZFNs, TALENs and CRISPR/Cas9
References
@BBCNews, (2015a), 'China to end one-child policy and allow two', BBC, available at http://www.bbc.com/news/world-asia-34665539, accessed on 16/10/2016
@BBCNews, (2015c), 'Forced sterilisation haunts Peruvian women decades on', BBC, available at http://www.bbc.com/news/world-latin-america-34855804, accessed on 16/10/2016
Allenspach, E., D. J. Rawlings, and A. M. Scharenberg, (2003), X-Linked Severe Combined Immunodeficiency, GeneReviews, available at https://www.ncbi.nlm.nih.gov/books/NBK1410/, accessed on 16/10/2016
Bartlett, J., (2014), 'Meet the transhumanist party: 'Want to live forever? Vote for me'', The Telegraph available at http://www.telegraph.co.uk/technology/11310031/Meet-the-Transhumanist-Party-Want-to-live-forever-Vote-for-me.html, accessed on 6/10/2016
Berg, P., (2008), 'Meetings that changed the world: Asilomar 1975: DNA modification secured', Nature, 455, 290-91
Bostrom, N., (2003a), 'Human Genetic Enhancements - A Transhumanist Perspective', Journal of Value Inquiry, 37 (4), 493-506
Bostrom, N., (2003b), 'Transhumanist values', in Adams, F.(ed.), Ethical Issues for the 21st Century, available at www.nickbostrom.com/ethics/values.html, accessed on 01/11/2016
Bostrom, N., (2004), 'Recent developments in the ethics, science, and politics of life-extension', Ageing Horizons, 3, 28-33, available at http://www.nickbostrom.com/ethics/life-extension.html, accessed on 6/10/2016
Calico, (2015), 'We're tackling aging, one of life's greatest mysteries', Calico, available at https://www.calicolabs.com, accessed on 24/04/2017
Cavazzana-Calvo, M., S. Hacein-Bey, G. de Saint Basile, F. Gross, E. Yvon, P. Nusbaum, F. (2000), 'Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease', Science, 288(5466), 669-72.
Cressey, D., A. Abbott, and H. Ledford, (2015), 'UK scientists apply for licence to edit genes in human embryos', Nature News, available at http://www.nature.com/news/uk-scientists-apply-for-licence-to-edit-genes-in-human-embryos-1.18394, accessed on 6/10/2016
Cyranoski, D., and S. Reardon, (2015), 'Chinese scientists genetically modify human embryos', Nature News, available at http://www.nature.com/news/chinese-scientists-genetically-modify-human-embryos-1.17378, accessed on 6/10/2016
Darnovsky, M., (2015a)', CGS: Open letter calls for prohibition on reproductive human germline modification', Center for Genetics and Society, available at http://www.geneticsandsociety.org/article.php?id=8999, accessed on 6/10/2016
Darnovsky, M., (2015b), 'Human gene editing is a social and political matter, not just a scientific one', The Guardian, available at https://www.theguardian.com/science/2015/dec/04/human-gene-editing-is-a-social-and-political-matter-not-just-a-scientific-one, accessed on 6/10/2016
Doudna, J., (2015), 'Perspective: Embryo editing needs scrutiny', Nature, 528, S6
Furness, H., (2013), 'Sir David Attenborough: Humans have stopped evolving', The Telegraph, available at http://www.telegraph.co.uk/news/science/evolution/10297124/Sir-David-Attenborough-Humans-have-stopped-evolving.html, accessed on 24/04/2017
Gaj, T., C. A. Gersbach, and C. F. Barbas, (2013), 'ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering', Trends in Biotechnology, 31, 397-405.
Geddes, L., (2013), ''Bubble kid' success puts gene therapy back on track', New Scientist, available at, https://www.newscientist.com/article/mg22029413-200-bubble-kid-success-puts-gene-therapy-back-on-track/, accessed on 6/10/2016
Gupta, R. M., and K. Musunuru, (2014), 'Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9', Journal of Clinical Investigation, 124 (10), 4154-61
Hacein-Bey-Abina, S., A. Garrigue, G. P. Wang, J. Soulier, A. Lim, E. Morillon, E. Clappier, L., Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M., (2008), 'Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1', Journal of Clinical Investigation, 118 (9), 3132-42
Harris, J., (2015), 'Why human gene editing must not be stopped', The Guardian, available at https://www.theguardian.com/science/2015/dec/02/why-human-gene-editing-must-not-be-stopped, accessed on 6/10/2016
Hesman Saey, T., (2015), 'Human gene editing research gets green light', ScienceNews, available at https://www.sciencenews.org/article/human-gene-editing-research-gets-green-light, accessed on 6/10/2016
HFEA, (2016), 'HFEA approves licence application to use gene editing in research', available at http://www.hfea.gov.uk/10187.html, accessed on 6/10/2016
Hinsliff, G., (2008), 'This couple want a deaf child. Should we try to stop them?', The Guardian, available at https://www.theguardian.com/science/2008/mar/09/genetics.medicalresearch, accessed on 6/10/2016
Honigsbaum, M., (2013), 'The future of robotics: in a transhuman world, the disabled will be the ones without prosthetic limbs', The Guardian, available at https://www.theguardian.com/technology/2013/jun/16/future-robotics-bionic-limbs-disabled, accessed on 6/10/2016
Ishino, Y., H. Shinagawa, K. Makino, M. Amemura, and A. Nakata, (1987), 'Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product', Journal of Bacteriology, 169, 5429-33
Jinek, M., K. Chylinski, I. Fonfara, M. Hauer, J. A. Doudna, and E. Charpentier, (2012), 'A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity', Science, 337, 816-21
Joung, J. K., and J. D. Sander, (2013), 'Innovation TALENs: A widely applicable technology for targeted genome editing', Nature Reviews Molecular Cell Biology, 14, 49-55
Ledford, H., (2015), 'CRISPR, the disruptor', Nature News, 522, 20
Liang, P., Y. Xu, X. Zhang, C. Ding, R. Huang, Z. Zhang, J. Lv, X. Xie, Y. Chen, Y. Li, Y. Sun, Y. Bai, Z. Songyang, W. Ma, C. Zhou, and J. Huang, (2015), 'CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes', Protein & Cell, 6, 363-72
Maude, S. L. N. F., P. Shaw, R. Aplenc, D.M. Barrett, N.J. Bunin, A. Chew, V. Gonzalez, Z. Zheng, S. F. Lacey, Y.D. Mahnke, J. J. Melenhorst, S. R. Rheingold, A. Shen, D. T. Teachey, B. L. Levine, C. H. June, D. L. Porter, and S. A. Grupp, (2014), 'Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia', New England Journal of Medicine, 371, 1507-17 New England Journal of Medicine, 371, 1507-17
Microsoft (2017), 'Artificial Intelligence', available at https://www.microsoft.com/en-us/research/research-area/artificial-intelligence/, accessed on 29/03/2017
Nemudryi, A. A., K. R. Valetdinova, S. P. Medvedev, and S. M. Zakian, (2014), 'TALEN and CRISPR/Cas Genome editing systems: Tools of discovery', Acta Naturae, 6, 19-40
ONPI, (2015), 'International summit on human gene editing: Statement of task', The National Academies of Sciences, Engineering, and Medicine, available at http://www.nationalacademies.org/gene-editing/gene_167925, accessed on 6/10/2016
Oxford University Press, (2017), 'Transhumanism definition entry', available at, https://en.oxforddictionaries.com/definition/transhumanism, accessed on 24/04/2017
Page, M. L., (2016), 'Gene editing saves girl dying from leukaemia in world first', New Scientist, available at https://www.newscientist.com/article/dn28454-gene-editing-saves-life-of-girl-dying-from-leukaemia-in-world-first/, accessed on 6/10/2016
Perez, E. E., J. Wang, J. C. Miller, Y. Jouvenot, K. A. Kim, O. Liu, N. Wang, G. Lee, V. V. Bartsevich, Y.-L. Lee, D. Y. Guschin, I. Rupniewski, A. J. Waite, C. Carpenito, R. G. Carroll, J. S. Orange, F. D. Urnov, E. J. Rebar, D. Ando, P. D. Gregory, J. L. Riley, M. C. Holmes, and C. H. June, (2008), 'Establishment of HIV-1 resistance in CD4(+) T cells by genome editing using zinc-finger nucleases', Nature Biotechnology, 26, 808-16
Qasim, W., P. Jal Amrolia, S. Samarasinghe, S. Ghorashian, H. Zhan, S. Stafford, K. Butler, G. Ahsan, K. Gilmore, S. Adams, D. Pinner, R. Chiesa, S. Chatters, S. Swift, N. Goulden, K. Peggs, A. Thrasher, P. Veys, and M. Pule, (2015), 'First clinical application of TALEN engineered universal CAR19 T cells in B-ALL', Blood, 126, 2046
Quinlan, A., (2015), 'CRISPR: The hopes, the fears, and the biology', Bioradiations, available at http://www.bioradiations.com/crispr-the-hopes-the-fears-and-the-biology/, accessed on 6/10/2016
Reardon, S., (2015a), 'Gene-editing summit supports some research in human embryos'. Nature News, available at http://www.nature.com/news/gene-editing-summit-supports-some-research-in-human-embryos-1.18947, accessed on 6/10/2016
Reardon, S., (2015b), 'Global summit reveals divergent views on human gene editing', Nature News, 528, 173
Reardon, S., (2015c), 'Leukaemia success heralds wave of gene-editing therapies', Nature News, 527, 146
Reardon, S., (2015d), 'NIH reiterates ban on editing human embryo DNA', Nature News, available at http://www.nature.com/news/nih-reiterates-ban-on-editing-human-embryo-dna-1.17452, accessed on 6/10/2016
Reardon, S., (2016), 'Ethics of embryo editing paper divides scientists', Nature News, available at http://www.nature.com/news/ethics-of-embryo-editing-paper-divides-scientists-1.17410, accessed on 6/10/2016
Sample, I., (2015), 'Future of human gene editing to be decided at landmark summit', The Guardian, available at https://www.theguardian.com/science/2015/nov/28/future-human-gene-editing-landmark-summit-dna-crispr-embyos, accessed on 01/11/2016
Sander, J. D., and J. K. Joung, (2014), 'CRISPR-Cas systems for editing, regulating and targeting genomes', Nature Biotechnology, 32, 347-55.
Sharp, G., (2008), 'Defining medical problems: Is deafness a disease?', Sociological Images, available at https://thesocietypages.org/socimages/2008/11/06/defining-medical-problems-is-deafness-a-disease/, accessed on 17/10/2016
Tebas, P., D. Stein, W. W. Tang, I. Frank, S. Q. Wang, G. Lee, S. K. Spratt, R. T. Surosky, M. A. Giedlin, G. Nichol, M. C. Holmes, P. D. Gregory, D. G. Ando, M. Kalos, R. G. Collman, G. Binder-Scholl, G. Plesa, W.-T. Hwang, B. L. Levine, and June, C. H., (2014), 'Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV', New England Journal of Medicine, 370, 901-10
The Francis Crick Institute, (2016), 'HFEA approval for new 'gene editing' techniques', The Francis Crick Institute, available at https://www.crick.ac.uk/news/science-news/2016/02/01/hfea-decision/, accessed on 6/10/2016
The National Academies of Sciences and Medicine, (2015), 'On human gene editing: A global discussion', available at http://www.nationalacademies.org/gene-editing/Gene-Edit-Summit/index.htm, accessed on 17/10/2016
Transhumanist Party, (2017), Transhumanist Party Principles, Transhumanist Party, available at http://www.transhumanistparty.org.uk, accessed on 24/04/2017
Sanders, R., (2015), Science magazine names CRISPR 'Breakthrough of the Year', University of California, available at https://www.universityofcalifornia.edu/news/science-magazine-names-crispr-breakthrough-year, accessed on 24/04/2017
Travis, J., (2015), 'Inside the summit on human gene editing: A reporter's notebook', Science, available at http://www.sciencemag.org/news/2015/12/inside-summit-human-gene-editing-reporter-s-notebook, accessed on 17/10/2016
United States Holocaust Memorial Museum, (2015), 'The biological state: Nazi racial hygiene, 1933-1939', available at https://www.ushmm.org/wlc/en/article.php?ModuleId=10007057, accessed on 6/10/2016
University of Leeds (2015), 'Leeds wins £4.2m funding to develop robot fixers of the future', available at https://www.leeds.ac.uk/news/article/3774/leeds_wins_42m_funding_to_develop_robot_fixers_of_the_future, accessed on 6/10/2016
Urnov, F. D., E. J. Rebar, M. C. Holmes, H. S. Zhang, and P. D. Gregory, (2010), 'Genome editing with engineered zinc finger nucleases', Nature Reviews Genetics, 11, 636-46.
Zayner, J., (2015), 'DIY CRISPR genome engineering kits', available at http://www.the-odin.com/diy-bacterial-gene-engineering-crispr-kit/, accessed on 17/10/2016
To cite this paper please use the following details: Hume, H. R. (2017), 'Gene editing: The road to transhumanism?', Reinvention: an International Journal of Undergraduate Research, Volume 10, Issue 1, http://www.warwick.ac.uk/reinventionjournal/archive/volume10issue1/hume. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal at warwick dot ac dot uk.