Applications
Despite recent advances in in vivo-directed evolution techniques and the interest they have attracted so far, their impact in applied biotechnology is limited because of their limitations in programmability, selective drivers, cost and scalability. Here, we propose to construct a general-purpose programmable evolution machine able to quickly evolve new biomolecules or phenotypes in bacterial cells. The proposed device will use existing phage technology and synthetic regulation to engineer a programmable directed evolution machine able to produce biomolecules or biocomputational functionality two orders of magnitude faster than conventional techniques, while consuming fewer consumables. In its core, living matter will be subject to combinatorial search algorithms that will exploit large numbers of small, separate, bacterial populations.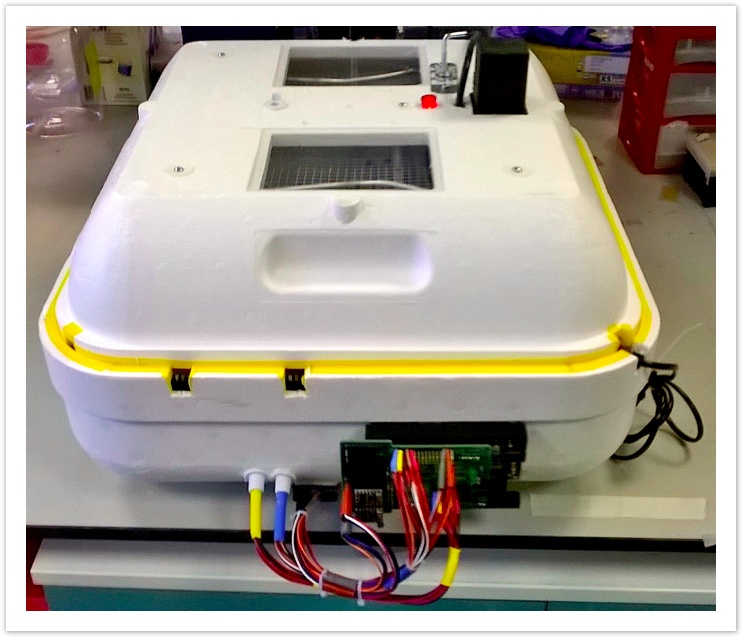
We present the development of Evoprog machines that enable the community of synthetic biologists and biochemists low cost experiments to evolve new biomolecules with desired activities or organisms with selectable phenotypes. Our objective was to build a universal evolution platform for do-it-yourself biologists who do not have background in engineering and have no resources to buy expensive equipment for continuous cultures. Our machines are thoroughly tested for robustness, and also run real biological experiments to validate the concept. The machine is assembled using low cost materials, it is scalable and expandable, is user friendly and allows for great experimental flexibility.
Critically, all the hardware we develop will be released along with detailed assembly manuals and operation guides. Our belief is that biologists can excel when engineering challenges and limitations of stiff hardware specifications can be removed. Evoprog aims for that.
Our international consortium works in synergy to convert conceptual idea of fluidic hardware, programming the controllers and biology to ensure the Evoprog machine earned its name and recognition in the next generation of biological experimentation where only limitation is posed by the experimenter.
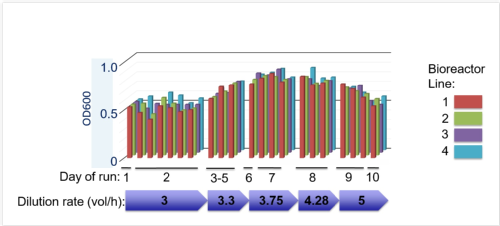
Evoprog machine v. 2.0 equipped with valves and sensors for the directed evolution experiments. [January 2016], and graph with monitoring the consistency of run parameters across four independent bioreactors for 10 days of uninterrupted run.
The newest version of our machine is able to run evolution experiments whilst maintaining and modulating selection constrains that are programmed the biologist.
Introduced hardware include arduino controller (with code nearly being ready for public release), operating shield and egg incubator with pre-installed heating module.
Periodical sample analysis allows to infer the status of the evolution.

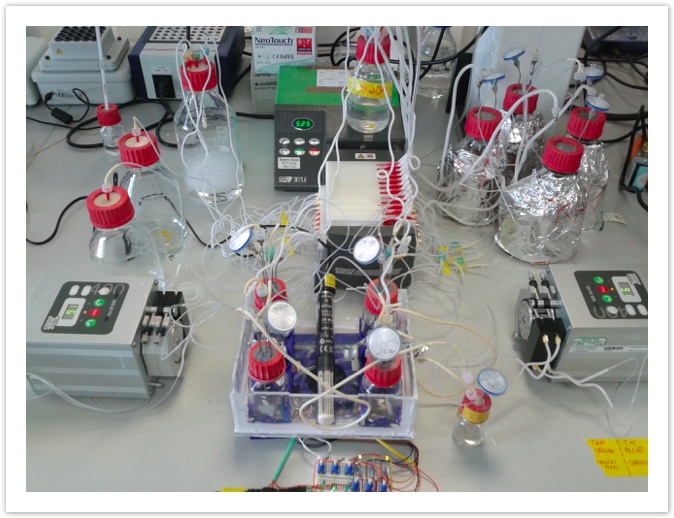
Our previous, simpler prototype of the accelerated evolution machine was also based on a egg incubator, which was a solid and cost effective housing. This setup consists of interconnected multi-bioreactor, temperature and flow rate controlled. [summer 2015]
Low cost materials include 3D printed holders, motor fans with fixed magnets serve as local mixers, tubing and bottles are commercially available.
Prototype implemented for using up to 6 bioreactors for directed evolution. [early summer 2015]. This hardware include a low cost temperature heater with probe that is commonly used in fish tanks.
This prototype was less advanced and the stability of the temperature control was harder to achieve. Despite, we succeeded with few key biological experiments using this setup.
 |
 |
Intermediate prototype in which customized heating and stirring system has been implemented along with 3D printed holders. The setup for the programmable directed evolution machine has been miniaturized and multiplied later. [spring 2015]

Next version of bioreactors system implemented for studying the dynamics of two bacterial
continuous cultures involved in directed evolution. A preliminary automated level (Arduino micro-controller) has been developed for controlling desired flow rate and temperatures. [early spring 2015]
This system included a commercial heater module that was later replaced with smaller and more suitable fish tank heater.
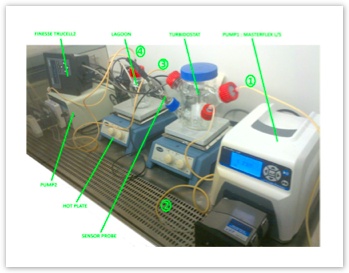
The very first tests of bioreactor setup for the continuous directed evolution. [winter 2014/2015].
In these tests we gathered common lab
equipment to make sure we can control the flow, monitor the bacterial growth and pre-optimize conditions.
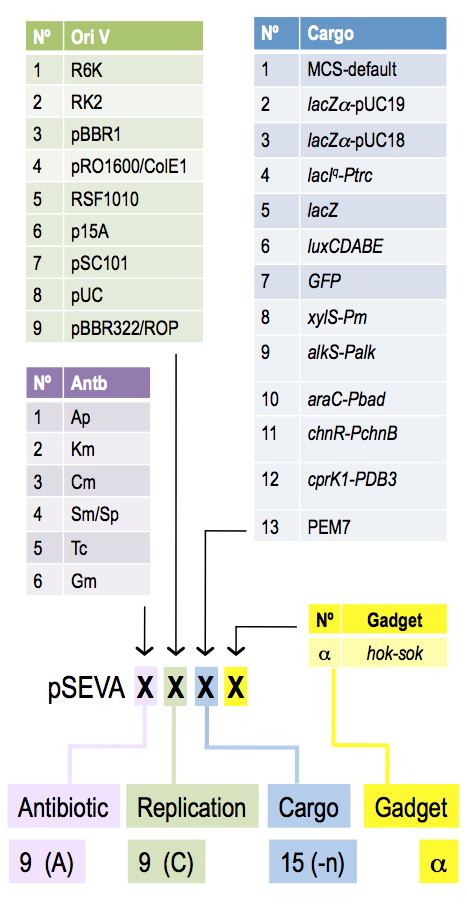
Implementation of genetic parts is performed using SEVA plasmid system developed by Victor de Lorenzo. Genetic elements are insulated by defined terminators and flanked by specific restriction sites, which allows for rapid exchange of elements between different constructs. All SEVA plasmids follow the same organization.
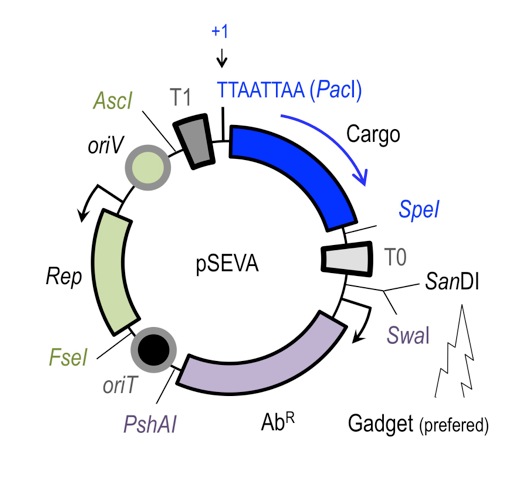
SEVA provides rich spectrum of origin of replications covering large range of copy numbers, and resistance markers.