WMG News
WMG charges ahead with battery research
WMG, at the University of Warwick, has received a share of £19 million from the Faraday Institution - the UK’s flagship institute for electrochemical energy storage research.
storage research.
The funding has been allocated to four key battery research projects aimed at delivering an impact for the UK. These existing projects across three different research areas — next generation cathode materials, electrode manufacturing and sodium-ion batteries — have been reshaped to focus on the areas with the greatest potential for success.
WMG is taking a key role in two of the four, reshaped projects entitled FutureCAT and Nextrode.
WMG’s Professor of Battery Innovation, Louis Piper, will now co-lead FutureCAT, a battery cathode research project, focusing on understanding novel redox processes as a route to stabilise both high capacity, high performance, nickel rich and emerging cathodes and scalable designer morphologies. The project will build on its success in developing reliable, scalable routes to deliver a longer lifetime, high-energy/power cathodes, essential for electric vehicles.
Whereas in Nextrode, a battery electrode project, WMG is one of six university partners, led by the University of Oxford, alongside six industry partners. Researchers at WMG will investigate ways to make electrodes for Li-ion batteries unlocking the electrochemical potential.
Professor Pam Thomas, CEO, Faraday Institution, commented: “The Faraday Institution remains steadfast in its commitment to identify and invest in battery research initiatives that hold the greatest potential for making significant societal, environmental, and commercial contributions. This announcement signals the completion of our latest round of project refocusing, enabling us to allocate even more effort towards those areas of research that offer maximum potential in delivering transformative impact.”
James Gaade, Research Programme Director commented: “We are pleased that the reshaping process has bolstered the capabilities and expertise of researchers on the four projects. The realignment includes a focus around research into sustainable manufacturing methods and materials, and the need to further develop and scale up manufacture of promising materials discovered in the first three years of the projects.”
Project information
FutureCat – High nickel content, high performance cathode materialsLink opens in a new window
FutureCat, co-lead by WMG’s Professor Louis Piper, and the University of Sheffield’s Professor Serena Cussen is targeting step-changes in:
- Understanding novel redox processes as a route to stabilise both high capacity, high performance, nickel rich and emerging cathodes. The project continues its focus on doped and dual-doped lithium nickel oxides (LNO) (both polycrystalline and single crystals), including use of protective coatings. The team will also investigate the use of polyanionic cathodes, use modelling to inform the search for new candidate materials, and research designer electrolytes with the intention of stabilising the interphase layer.
- Scalable designer morphologies. The project will build on its success with doped-LNO in developing reliable, scalable routes to deliver a longer lifetime, high-energy/power cathodes through the use of gradient morphologies, co-doped cathodes (with the aim of delivering reversible discharge capacities exceeding 220 mAh/g), single crystal particles and thin coatings.
- Materials delivery: The scale up of the high nickel W-LNO material previously developed by FutureCat is being transferred to the Degradation project for testing in industry-relevant pouch cells. FutureCat will continue to investigate the manufacturing scale-up of other Ni-rich cathode materials, down-selecting promising active materials based on earth-abundant elements. Research includes the use of laser patterning to increase power densities, investigation of cracking as a failure mechanism to determine routes to resilient cathode manufacture, atomic layer deposition of coatings to improve electrode longevity, and optimisation of cycle life through the use of electrically conductive binders.
Nextrode – electrode manufacturingLink opens in a new window
Nextrode is focused on researching, understanding and quantifying the potential of smart electrode manufacturing to reduce manufacturing costs and improve the performance of batteries. Benefits could be realised in both mature material systems already used commercially and in new emerging high performance battery systems. The project is developing new practical manufacturing innovations – including traditional slurry cast electrodes and novel low or no solvent electrodes – that could deliver the benefits of smart electrodes to the industrial scale and improve sustainability of processes.
The project is researching the underpinning manufacturing science that could alleviate constraints in electrode manufacturing through engineering particle design and improved understanding of the relationship between powder properties and deposition/calendering techniques. Nextrode is designing manufacturing process steps and using advanced in-line measurements to enable slurry casting to be brought under closed-loop control. Researchers are manufacturing new arrangements of anode and cathode materials, identifying conditions where benefits are maximised and developing cells that expand the energy-power-lifetime design space.
The new phase of research projects described will progress over the two years from 1 October 2023 to 30 September 2025.
Find out more about WMG’s Electrochemical Engineering research here: Electrochemical Materials (warwick.ac.uk)
For more information on the Faraday Institution, visit www.faraday.ac.ukLink opens in a new window
£1.5m funding secured to advance the investigation of microstructures in battery materials
Researchers at WMG, University of Warwick’s Forensics & Advanced Characterisation of Batteries Group, have secured £1.5m funding for materials analysis in multiple format batteries.
The funding, from the University of Warwick’s Academic Equipment Fund and the High Value Manufacturing Catapult, will be used to purchase a Plasma Focused Ion Beam (PFIB) microscope. The microscope is key to the accelerated development of new battery chemistries, providing unique access to the critical interfaces within battery cells that dictate best performance. This will be instrumental in developing new materials for better batteries, regardless of their end use application.
Focused Ion Beam (PFIB) microscope. The microscope is key to the accelerated development of new battery chemistries, providing unique access to the critical interfaces within battery cells that dictate best performance. This will be instrumental in developing new materials for better batteries, regardless of their end use application.
This PFIB will be the first specifically configured microscope dedicated to battery research in the world, allowing researchers at WMG to inform battery manufacturing, answer key scientific questions and link with industry and growing supply chains.
There is increased recognition in the battery community that the integration of new chemistries needed for the UK Government’s 2030 electric vehicle battery targets will require integrating new manufacturing processes with advanced microscopic characterisation. The PFIB has been specifically designed to address the critical challenges of studying alkali-based battery systems and will provide unique insights needed for the development of next generation batteries.
 The performance of battery materials is dictated by the stability, efficiency and functionality of the interfaces, i.e. the solid electrolyte interphase (SEI) at the anode and oxygen-induced cathode-electrolyte interface (CEI) at the cathode. Attempts to analyse these interfaces, in order to determine structure and chemistry, is seriously compromised using conventional techniques by the extreme air-sensitivity, beam sensitivity and the high volatility of certain species present. The specially configured PFIB microscope will address these issues.
The performance of battery materials is dictated by the stability, efficiency and functionality of the interfaces, i.e. the solid electrolyte interphase (SEI) at the anode and oxygen-induced cathode-electrolyte interface (CEI) at the cathode. Attempts to analyse these interfaces, in order to determine structure and chemistry, is seriously compromised using conventional techniques by the extreme air-sensitivity, beam sensitivity and the high volatility of certain species present. The specially configured PFIB microscope will address these issues.
WMG is one of seven HVM Catapult centres in the UK and is the lead centre for transport electrification. Investment in this PFIB is part of a range of equipment investments by the HVM Catapult and the University of Warwick to maintain WMG’s leading position in battery technology.
The PFIB has already secured interest from the Faraday Institution and from the Royal Academy of Engineering’s Lord Bhattacharyya Education Programme. Starting in the Autumn, a student will use this for a project entitled “The development of a new multi-modal capability for investigating the performance-controlling interfaces and microstructures that underpin operation of battery materials.”
The Lord Bhattacharyya Education Programme provides up to 90 bursaries annually for local students from lower socio-economic backgrounds. The objectives for the scheme include encouraging a greater number of young people from a more diverse range of backgrounds, raising their aspirations and skill levels. Moreover, it supports the growth of a science and engineering skills base for the UK.
The project will make extensive use of the new system to develop strategies for studying the degradation of buried interfaces and structure dynamics in state-of-the-art high Ni NMC cathodes as a function of cycle ageing i.e., the evolution of the cathode-electrolyte interphases. The platform provides some unique opportunities for developing powerful new ways to characterise these controlling interfaces and will form the basis for the project. Preliminary research will commence in October 2023. The project will be advertised online for interested applicants to apply – the studentships page, Jobs.ac.uk, FindAPhD.com and the Doctoral College website.
Find out more about WMG’s electrochemical research here: Electrochemical Materials (warwick.ac.uk)
The new PFIB microscope will be based in WMG’s Advanced Material Manufacturing Centre (AMMC).
Battery technology research collaboration initiated between OXECO and WMG, the University of Warwick
- OXECO and WMG at the University of Warwick will conduct a 15-month research programme into lithium-ion battery coatings
- Research is expected to advance next generation active materials and coating foils used to create electrodes
- The partnership aims to improve lithium-ion battery performance, longevity, and manufacturing
 OXECO, a spin-out of the University of Oxford, has partnered with WMG at the University of Warwick, for a 15-month collaboration on lithium-ion battery technology. The partnership aims to advance the lithium-ion battery industry by leveraging WMG’s research on battery cell development and optimisation, alongside OXECO’s unique technology platform.
OXECO, a spin-out of the University of Oxford, has partnered with WMG at the University of Warwick, for a 15-month collaboration on lithium-ion battery technology. The partnership aims to advance the lithium-ion battery industry by leveraging WMG’s research on battery cell development and optimisation, alongside OXECO’s unique technology platform.
The research programme aims to enhance the performance, longevity, and ease of manufacturing of lithium-ion batteries by focusing on the preparation of active materials and coating foils used to create electrodes.
The programme is expected to yield transformative results in the improvement of current lithium-ion batteries and be a significant step towards the development of more efficient, reliable, and sustainable energy storage solutions.
This partnership will further OXECO’s aim to leverage innovative surface chemistry to accelerate our net-zero future. Jon-Paul Griffiths, founder and Chief Technology Officer at OXECO, commented that: “This is a remarkable opportunity to help steer the progress of battery technology - a crucial industry for the realisation of a sustainable energy economy. WMG has exceptional proficiency and credibility in battery research, coupled with the ability to manufacture from bench to pilot scale.
"This marks the initial phase of our efforts towards the integration of ONTO™ chemistry into lithium-ion batteries, and we are also delving into other domains of our chemistry for diverse applications in batteries, such as separator membranes. Our team is dedicated to forging a path towards cutting-edge technological advancements that will shape the future of energy storage solutions.”
WMG has an international reputation for working successfully with industry, with a history of partnering with pioneering entities, including Lotus, Aston Martin, JLR, BAE Systems, IBM, and Plug and Play, as well as the latest technology innovators.
Dr Mark Copley, Chief Engineer in WMG's Electrochemical Materials and Manufacturing team, concluded: “We are excited to be partnering with OXECO. This collaboration will draw on the University’s extensive expertise in battery technologies and OXECO’s chemistry to improve battery performance and longevity and help the global transition to sustainable power solutions through innovative research and training.”
About OXECO
OXECO is a chemistry technology company that designs, develops, and manufactures thin coatings and materials for the transport and clean technology sectors. The company’s core innovative technology controls the way that surfaces behave using cutting-edge carbene chemistry. This technology was born in the University of Oxford’s Department of Chemistry and developed over more than two decades.
Pushing the limits of battery research with nickel-rich chemistries
New research has shown that understanding how oxygen participates in energy storage is critical for developing higher energy density batteries, in a new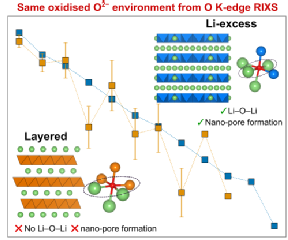 paper published by experts at WMG, at the University of Warwick.
paper published by experts at WMG, at the University of Warwick.
Using advanced X-ray techniques, researchers at WMG, together with the Faraday Institution's FutureCat consortium, have obtained new insights into the oxygen redox activity in conventional ni-rich cathodes, which will help to deliver improved electric vehicle performance.
Range anxiety is a key concern of many potential EV buyers, but range is steadily improving as battery technology and research evolves. The Faraday Institution’s Next Generation Lithium-Ion Cathode Materials project, FutureCat, aims to develop understanding of existing and newly discovered cathode chemistries to deliver improved EV performance, whilst considering sustainability.
Professor Louis Piper, from WMG at the University of Warwick, who led the research explained: “Transitioning to electrification requires integrating advanced materials science into battery processing to develop cheaper, safer, faster and better batteries, which is the focus of our research.”
The battery field is moving to increasing nickel contents in cathodes to meet the Government’s stringent EV 2030+ targets. These roadmaps assume successful strategies in material development to allow cathodes like W-LNO to operate at high voltages without degrading. This work provides the platform towards realising that goal by better understand the redox mechanisms (i.e., the reactions that enable charging/discharging the battery) at high voltage operation.
The study employed advanced x-ray characterisation techniques at the Diamond Light Source in Oxford and at WMG. The team at WMG utilised novel in-house x-ray absorption spectroscopy which enabled researchers to look at the electrode redox process of the battery cathodes after careful disassembly. Researchers were surprised to find that the oxidised oxygen species had the same characteristics as another group of Li-ion battery cathodes, Li-excess transition metal oxides. Reconciling how the same oxidised oxygen environment exists in both conventional and Li-excess cathodes is critical for unlocking how to develop the next generation of cathodes.
Professor Piper adds: “This work highlights how large-scale collaborative fundamental studies are needed even for supposedly ‘known’ systems.”
WMG will be continuing with further studies in this field, supported by the Faraday Institution, for the benefit of cathode battery manufacturers.
A link to the published article can be found here:
https://journals.aps.org/prxenergy/pdf/10.1103/PRXEnergy.2.013005
WMG in landmark battery development partnership
Researchers at WMG at the University of Warwick, are part of a unique four-way partnership, with Addionics, technology innovation catalyst CPI and James Durrans Group, which will position the UK as a technology hub for global battery development.
James Durrans Group, which will position the UK as a technology hub for global battery development.
Project Constellation is an extension of Project STELLAR which focused on improving battery power and cycle life. Project Constellation takes the research to the next level addressing improvements to battery performance, which will in turn lower development and production costs.
The team at WMG will use its expertise in pilot scale electrode production, cell manufacturing and electrochemical testing to support and de-risk rapid technology screening and accelerate the route to market.
Farid Tariq Ph.D, CTO and Co-founder of Addionics, explains:" Constellation builds on the success of Stellar taking it beyond basic tests and towards industrially relevant scales. We are excited because it provides a strong integration piece of our technology with world leaders in coating and fabrication, and active material fabrication (WMG, CPI, James Durrans) that can show how our very smartly designed and structured current collectors can fit into a viable battery ecosystem and provide benefits from our technology. This is readily transferable knowledge and will push the creation of new methods to overcome modern limitations of batteries and fabrication."
Mark Copley, Chief Engineer in WMG at the University of Warwick’s Electrochemical Materials and Manufacturing team said: “WMG is delighted to be a partner in the CONSTELLATION consortium. Utilising our experience in scaling up new technologies, from lab to pilot line, we feel that we will be able to further the development of Addionics’ current collector technology whilst coupling in Durrans’ graphite and formulation developments, as derived by CPI.
“The project goals fit very well with the ideals of WMG, which is to work collaboratively with industry to deliver high-quality, applied, research and development. We look forward to the results that will be generated through this funded collaborative effort.
Project Constellation is a two year project, funded by the UK Government’s Faraday Institution’s Faraday Battery Challenge Round 5 Innovation.
About the partners
Addionics
Addionics is a next-generation battery technology company revolutionizing battery performance through its chemistry-agnostic Smart 3D Electrode architecture. The company’s scalable, cost-effective manufacturing process combined with its AI-based optimization software significantly improves the performance of any kind of chemistry, achieving batteries with higher energy density, faster charging, and longer lifetime, at low cost. With the mission to accelerate an electrified economy and decarbonized future, Addionics is unlocking the full potential and accelerating the electrification revolution through its drop-in solutions.
CPI
We take great ideas and inventions, and we make them a reality. Born in the North East of England in 2004, CPI is an independent deep tech innovation organisation and a founding member of the High Value Manufacturing Catapult.
We're a team of intelligent people using advances in science and technology to solve the biggest global challenges in healthcare and sustainability. Through our incredible people and innovation infrastructure, we collaborate with our partners in industry, academia, government, and the investment community to accelerate the development and commercialisation of innovative products.
Our work ranges from health technologies, advanced drug delivery systems, and medicines manufacturing innovations for multiple modalities including small molecules, biologics, and nucleic acids; to developing sustainable materials for energy storage and packaging, as well as novel food, feed, and nutraceuticals, that are all underpinned by digital technology. We turn the entrepreneurial spirit and radical thinking of our people and partners into incredible impact that makes our world a better place.
Let’s innovate together: uk-cpi.comLink opens in a new window
Connect with us: LinkedIn TwitterLink opens in a new window InstagramLink opens in a new window FacebookLink opens in a new window
James Durrans Group
Long established family owned manufacturing company (1863) based in Penistone near Sheffield but with manufacturing sites across the globe. We provide pro-active solutions to our customer needs. Experts in carbon processing and technology and the manufacture of heat resistant coatings and graphitic dispersions.
WMG battery experts publish Faraday Insight addressing battery degradation
 WMG Associate Professor Dr Mel Loveridge, and Principal Engineer Martin Dowson, have authored a special Faraday Insight entitled “Why Batteries Fail and How to Improve Them: Understanding Degradation to Advance Lithium-Ion Battery Performance,” a key consideration as we transition to a fully electric future.
WMG Associate Professor Dr Mel Loveridge, and Principal Engineer Martin Dowson, have authored a special Faraday Insight entitled “Why Batteries Fail and How to Improve Them: Understanding Degradation to Advance Lithium-Ion Battery Performance,” a key consideration as we transition to a fully electric future.
It is part of a collection, of pieces from leading UK battery experts, published by the Faraday Institution. The Faraday Insights are evidence-based assessments of the market, economics, commercial potential, and capabilities for energy storage technologies. The insights are concise briefings that aim to help bridge knowledge gaps across industry, academia, and government.
The briefing addresses key requirements in lifetime, performance and safety needed for LIBs to continue to lead electrification in multiple sectors, and explains why, to achieve this researchers need to better understand – and find ways to mitigate – the many causes of battery degradation.
Read the Insight here: www.faraday.ac.uk/get/insight-10/
Read more about WMG’s Electrochemical Engineering research here: Electrochemical Materials (warwick.ac.uk)
New insight into how lithium-rich cathode materials for high energy EV batteries store charge at high voltages
- By 2030 only EV’s will be in production, meaning manufacturers are racing to create a high-energy battery that’s affordable and charges efficiently, but conventional battery cathodes cannot reach the targets of 500Wh/Kg
- Lithium-excess cathodes offer the ability to reach 500Wh/Kg but unlocking their full capacity means understanding how they can store charge at high voltages
- A new X-ray study lead by WMG, University of Warwick has resolved how the metals and oxygen facilitate the charge storage at high voltages
High energy storage batteries for EVs need high capacity battery cathodes. New lithium-excess magnesium-rich cathodes are expected to replace existing nickel-rich cathodes but understanding how the magnesium and oxygen accommodate charge storage at high voltages is critical for their successful adaption. Research led by WMG, University of Warwick in collaboration with U.S. researchers employed a range of X-ray studies to determine that the oxygen ions are facilitating the charge storage rather than the magnesium ions.
 Electric vehicles will one day dominate UK roads and are critical for eliminating CO2 emissions, but a major issue car manufacturers face is how to make an affordable long-lasting energy-dense battery that can be charged quickly and efficiently. There is therefore a race to make EV batteries with an energy storage target of 500 Wh/Kg, but these targets are not possible without changing to new cathode materials.
Electric vehicles will one day dominate UK roads and are critical for eliminating CO2 emissions, but a major issue car manufacturers face is how to make an affordable long-lasting energy-dense battery that can be charged quickly and efficiently. There is therefore a race to make EV batteries with an energy storage target of 500 Wh/Kg, but these targets are not possible without changing to new cathode materials.
Although progress has continued over the last 10 years to push the performance of state-of-the-art nickel-rich cathodes for EV, the material is unable to provide the energy density needed. To increase the capacity more lithium needs to be used, which means going beyond the ability of nickel to store electron charge.
Lithium-excess magnesium-rich cathodes offer sufficient energy density but to reach ultimately reach energy storage targets of 500Wh/Kg we need to understand how the electron charge is stored in the material. Simply put, is the electron charge stored on the magnesium or oxygen sites.
In the paper, ‘Whither Mn Oxidation in Mn-Rich Alkali-Excess Cathodes?’, published in the Journal ACS Energy Letters today the 17th of February, researchers from WMG, University of Warwick have overcome a significant milestone in understanding of charge storage in lithium-excess magnesium-rich cathodes.
Li-excess compounds that involve conventional and non-conventional redox, conventional refers to metal ions changing their electron density. Reversibly changing the electron density on the oxygen (or oxygen redox) without it forming O2 gas is unconventional redox. Various computational models exist in the literature describing different mechanisms involving both, but careful x-ray studies performed while the battery is cycling (operando) are ultimately required to validate these models.
Researchers between the UK and US, led by WMG at the University of Warwick, performed operando x-ray studies to precisely quantify magnesium and oxygen species at high voltages. They demonstrated how x-ray beams could irreversibly drive highly oxidized magnesium (Mn7+) to trapped O2 gas irreversibly in other materials.
However, by performing careful operando x-ray studies that circumvented beam damage and observe only trace amounts of Mn7+ forming upon charging in Li-excess cathodes during battery cycling.
Professor Louis Piper, from WMG, University of Warwick explains:
“We have ultimately resolved that oxygen rather than metal redox is driving the higher capacity, which means we can now design better strategies to improve cycling and performance for this class of materials.”
ENDS
17 FEBRUARY 2021
NOTES TO EDITORS
High-res images available at:
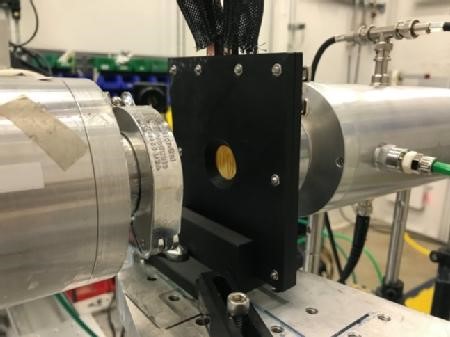
https://warwick.ac.uk/services/communications/medialibrary/images/february_2021/operando_x-ray_louis_piper.jpg
Caption: Photo of the operando x-ray studies being performed at a Synchrotron facility.
Credit: WMG, University of Warwick
Paper available to view at: https://pubs.acs.org/doi/10.1021/acsenergylett.0c02418
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
High-rate Li-ion batteries demonstrate superior safety
As the inevitable growth of transport electrification continues, the types of batteries that will be used in such vehicles, their charging parameters, infrastructure and timeframes are key considerations that will speed up the transition to electrification.
In the paper, ‘Determining the Limits and Effects of High-Rate Cycling on Lithium Iron Phosphate Cylindrical Cells’ published in and on the cover of the Journal Batteries, researchers from WMG, University of Warwick investigated the impacts on battery cell ageing from high current operation using commercial cells.
They used two tests to establish the maximum current limits before cell failure and applied this maximum current until cell failure. Testing was performed to determine how far cycling parameters could progress beyond the manufacturer’s recommendations.
During testing, current fluxes were increased up to 100 C cycling conditions. Charge and discharge current capabilities were possible at magnitudes of 1.38 and 4.4 times, respectively, more than that specified by the manufacturers’ claims. This increased current was applied for 500 charge-discharge.
However, the application of these currents resulted in a rapid decrease in capacity in the first 60 cycles as well as an increase in resistance. Furthermore, the application of such currents resulted in the increase of cell temperature, during both charge and discharge with natural convection during the rest step cooling the cell. Batteries operate in an optimum temperature range, and any deviations outside this can cause components and chemicals to start decomposing inside them.
They also identified deformation of the “jelly roll” (coiled electrodes and separator) with formation of lithium plating from testing and ageing. These deformations emanate from the centre of the cell in an axial direction towards the outside of the cell, suggesting the core of the cell was the hottest.
 Lead Engineer, Justin Holloway, from WMG, University of Warwick comments:
Lead Engineer, Justin Holloway, from WMG, University of Warwick comments:
“The testing showed there is a window for operating batteries above manufacturer stated current limits, however, whilst maintaining manufacturer stated voltage limits. We need to ensure that batteries operate in as the safest manner possible, and for an appropriate practical lifetime, which is why the manufacturers have these limits.
“We also identified thermal fatigue as the driving mechanism for jelly roll deformation. With each cycle of charge and discharge, the cell experienced thermal stresses causing deformation of its components. These deformations grew progressively with cycle number, while the jelly roll was constrained mechanically by the rigid outer can and centre pin.
“If convection cooling could be applied to the centre of the cell where the cell was the hottest, these deformations could be mitigated and controlled, allowing the cell to maintain capacity and resistance criteria for longer.”
The researchers would like to thank all involved in this work, including WMG’s High Value Manufacturing Catapult and The Faraday Institution. WMG’s Battery Forensic Group, led by Dr Mel Loveridge is keen to engage with industry and academia alike to grow advances in understanding new materials, battery performance and degradation modes.
ENDS
14 DECEMBER 2020
NOTES TO EDITORS
High-res images available at:
https://warwick.ac.uk/services/communications/medialibrary/images/december_2020/justin_holloway.jpg
Caption: Justin Holloway, from WMG, University of Warwick
Credit: WMG, University of Warwick
Paper available to view at: https://www.mdpi.com/2313-0105/6/4/57/pdf
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
The ultimate conditions to get the most out of high-nickel batteries
It is common knowledge in battery manufacturing that many cathode materials are moisture sensitive. However, as the popularity of high nickel-based battery components increases, researchers from WMG, University of Warwick have found that the drier the conditions that these cathodes are stored and processed in, then significant improvement in performance of the battery is gained.
High-Ni (Nickel) batteries are becoming increasingly popular worldwide, with more automotive companies investigating the use of high-Ni batteries for electric vehicles. 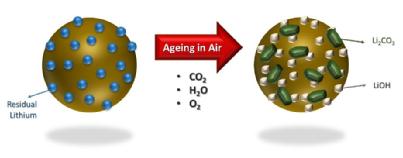 However, high-Ni cathode materials are prone to reactivity and instability is exposed to humidity, therefore how they are stored in order to offer the best performance is crucial.
However, high-Ni cathode materials are prone to reactivity and instability is exposed to humidity, therefore how they are stored in order to offer the best performance is crucial.
In the paper, ‘The effects of Ambient Storage Conditions on the Structural and Electrochemical Properties of NMC-811 Cathodes for Li-ion batteries,’ published in the journal Electrochimica Acta, researchers from WMG, University of Warwick propose the best way to store high-nickel cathodes in order to mitigate premature degradation.
Researchers exposed NMC-811 (high-Ni cathode material) to different temperatures and humidities, then measured the material’s performance and degradation in a battery over a 28 day period, analysing them using a combination of physical, chemical and electrochemical testing. This included high-resolution microscopy to identify the morphological and chemical changes that occurred at the micron and sub-micron scale during the batteries charging and discharging.
The storage conditions included vacuum oven-dried, as exposed (to humidity) and a control measure. Researchers looked for surface impurities, which include carbonates and H2O, and found there were three processes that can be responsible for impurities, including:
1. Residual impurities emanating from unreacted precursors during synthesis
2. Higher equilibrium coverage of surface carbonates/hydroxides (present to stabilise the surface of Ni-rich materials after the synthesis process)
3. Impurities formed during ambient storage time
They found that in all conditions, (oven dried and as-exposed) showed inferior first discharged specific capacity and cycling performance, compared to the control. However the as-exposed measure showed that after 28 days of ambient moisture exposure the H2O and CO2 react with the Li+ ions in the battery cell, resulting in the formation of lithium carbonate and hydroxide species.
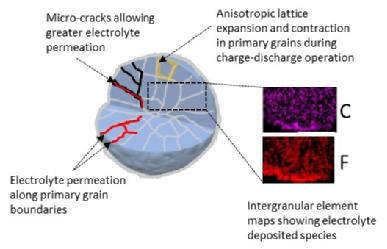 The formation of carbonates and oxides on the surface of NMC-811 contribute to the loss of the electrochemical performance during ageing of the materials, due to the inferior ionic and electronic conductivity, as well as the electrical isolation of the active particles. This means that they can no longer reversibly store lithium ions to convey “charge”. SEM analysis confirmed the inter-granular porosity and micro-cracks on these aggregate particles, following the 28 days of ambient exposure.
The formation of carbonates and oxides on the surface of NMC-811 contribute to the loss of the electrochemical performance during ageing of the materials, due to the inferior ionic and electronic conductivity, as well as the electrical isolation of the active particles. This means that they can no longer reversibly store lithium ions to convey “charge”. SEM analysis confirmed the inter-granular porosity and micro-cracks on these aggregate particles, following the 28 days of ambient exposure.
They can therefore conclude that the driest conditions, at dew points of around -45 oC, are the best for storing AND processing the materials, in order to then produce the best battery performance. Humidity conditions and exposure at junctions along the manufacturing process will cause the materials and components to experience; this results in shorter battery lifespan.
Dr Mel Loveridge from WMG at the University of Warwick comments:
“Whilst moisture is well known to be problematic here, we set about to determine the optimal storage conditions that are required to mitigate unwanted, premature degradation in battery performance. Such measures are critical to improve processing capability, and ultimately maintain performance levels. This is also of relevance to other Ni-rich systems e.g. NCA materials.”
Professor Louis Piper from WMG at the University of Warwick adds:
“Considerable global research effort will continue to focus on these materials, including how to protect their surfaces to eliminate risks of parasitic reactions prior to incorporation into electrodes. In the UK, leading research by the Faraday Institution has a project consortium entirely devoted to unravelling the degradation mechanisms of such industry-relevant materials.”
ENDS
18 NOVEMBER 2020
NOTES TO EDITORS
High-res images available at:
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/the_effects_of_ambient_air_storage_on_the_surface_stability_of_nmc-811.jpg
(1) Caption: The effects of ambient air storage on the surface of NMC-811
Credit: WMG, University of Warwick
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/post-mortem_nmc811_particle.jpg
(2) Caption: a-b) Post-mortem NMC811 particle, with no prior exposure to moist air, analysed by FIB-SIMS, targeting Lithium detection. c-d) Post-mortem NMC811 particle, after 28 days exposure to moist air, analysed by FIB-SIMS, targeting Lithium detection.
Credit: WMG, University of Warwick
https://warwick.ac.uk/services/communications/medialibrary/images/october_2020/schematic.jpg
(3) Caption: Schematic illustration of particle breakdown during charge-discharge of a battery
Credit: WMG, University of Warwick
Paper available to view at: https://www.sciencedirect.com/science/article/pii/S0013468620317515?via%3Dihub
New research says Sodium-ion batteries are a valid alternative to Lithium-ion batteries
- Lithium (Li)-ion batteries (LIBs) are the electrochemical energy storage systems of choice for a wide variety of applications, however other types of emerging battery technologies are currently on the path to share their dominant position.
- Among them Sodium (Na)-ion batteries (NIBs) have great potential to represent the next generation low cost and environmentally friendly energy storage solution. The diverse key performance indicators required by different applications and the market diversification is the driving force pushing the Na-ion technology closer to the market.
- A team of scientists including WMG at the University of Warwick combined their knowledge and expertise to assess the current status of the Na-ion technology from materials to cell development, offering a realistic comparison of the key performance indicators for NBs and LIBs.
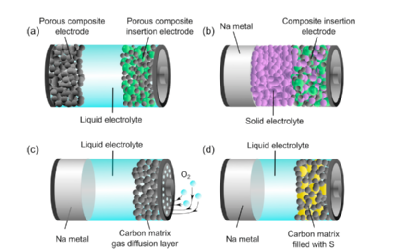 LIBs play a primary role in the transition to a low carbon economy. However, as the market rapidly expands, the environmental and social challenges associated with the mass production of LIBs is triggering large attention toward the search for alternative energy storage solutions based on materials that can be sourced in a sustainable and responsible way. In this scenario, NIBs represent an alternative low cost, sustainable and more environmentally friendly energy storage technology.
LIBs play a primary role in the transition to a low carbon economy. However, as the market rapidly expands, the environmental and social challenges associated with the mass production of LIBs is triggering large attention toward the search for alternative energy storage solutions based on materials that can be sourced in a sustainable and responsible way. In this scenario, NIBs represent an alternative low cost, sustainable and more environmentally friendly energy storage technology.
In the paper ‘Challenges of today for Na-based batteries of the future: from materials to cell metrics,' published on the 18th of September 2020 in the Journal of Power Sources, a large team of Na-ion technology expert scientists, led by WMG, at the University of Warwick (UK) analyse the prospect of NIBs taking a spot in the energy storage market. The paper also includes researchers from: Helmholtz Institute Ulm (Germany), College de France (France), Humboldt University Berlin (Germany), Institute for Energy technology (Norway), Université de Picardie Jules Verne (France), University of Bordeaux (France) and CIC energiGUNE (Spain).
Na- based batteries offer a combination of attractive properties. They are low cost, use sustainable precursors and have secure raw material supplies. In addition, they are considered as a drop-in technology which could benefit from the already existing Li-ion batteries manufacturing facilities.
As Li-based systems, Na-based batteries come in different forms, such as Na-ion, Na-all-solid-state-batteries, NaO2 and Na/S. While the last ones are seen as disruptive future technologies, the Na-ion technology represent an attractive technology almost ready to challenge the Li-ion batteries in specific applications.
Performance metrics are of utmost importance for the SIB technology to ensure a competitive cost per Wh and find a place in the market. In this work, the most promising electrode materials and electrolyte systems have been reviewed and performance metrics from the academic literature have been used to extrapolate full sodium ion cells performance indicators.
Authors indicate that with the ongoing development, the present best materials available for Na-ion cells should allow approaching the energy density of the present generation of Li-ion commercial cells. One of the most important application field for the developed sodium-ion battery prototypes is certainly stationary energy storage systems, where cost and cycle life represent two fundamental parameters. “In this field sodium-ion batteries have the potential to dominate the future market representing the most promising system to fill the gap between energy production and utilization by securing energy supply. However high-power applications in the electrified automotive field are a potential niche field application for NIBs” says Dr Ivana Hasa, Assistant Professor at WMG.
Further technological improvements are needed to increase the performance especially in terms of energy density. Extremely encouraging results have been achieved for the Na-ion technology in a very short time when compared to the Li-ion technology. Technological improvement will be achieved by cell component fabrication/assembly optimization, as occurred in the last thirty years for the LIB technology.
 Dr Ivana Hasa, from WMG, University of Warwick comments:
Dr Ivana Hasa, from WMG, University of Warwick comments:
“From an applied research point of view, the future research efforts should be devoted on fundamental research, materials discovery and understanding of the thermodynamic and kinetic processes governing the chemistry of these systems. In addition, the investigation of upscaled Na-ion batteries is of primary importance to obtain realistic data to benchmark the progress of the technology as well as the adoption of a common reporting methodology in the scientific community enabling a fair comparison among performance results.”
