The Effect of Visitor Density on the Behaviour of Two Siberian Tigers (Panthera tigris altaica) Housed in a Zoo Enclosure: A Case Study
Zoë Goldsborough[1], Department of Biology, University College Roosevelt
Abstract
Visitor density is often considered to have an effect on zoo animals, especially on more social species. However, the effect on solitary species, such as felids, could be even greater, since they are unaccustomed to interaction with other animals. This article investigated the effect of visitor density on a male and a female Siberian tiger housed at Dierenpark Amersfoort, the Netherlands. Their behaviour was observed over the course of 12 weeks on a weekly basis and average visitor count was calculated per five-minute observation period. Separate analyses were conducted per time of day and individual. The tigers were found to vary strongly in their behaviour and stress response. The average number of visitors present increased the amount of aggressive behaviour displayed by the female, although this effect was only significant in the Morning. The male showed stereotypic behaviour when visitor counts increased in the Morning and Midday. However, since visitor count is correlated to temperature it is difficult to separate their effect. The results of this article indicate that the female at least was affected by the presence of visitors, as she showed more aggressive behaviour towards the male when visitor counts were high, independent of weather.
Keywords: Visitor density, Panthera tigris altaica, animal behaviour, zoo animals, stereotypy, aggression.
Introduction
One of the best qualities of zoos is that animals can be observed up close in a safe and controlled setting, which holds many advantages but also presents challenges (Fernandez et al., 2009). One of these challenges lies in the effect of the visitors on the captive animals, called the visitor effect. There are three hypotheses concerning this effect. Firstly, visitors could be a type of social enrichment for the animals, presenting a source of change in an otherwise quite fixed environment (Chamove and Moodie, 1990; Hosey, 2000; Morris, 1964). Secondly, because captive animals are exposed to visitors throughout their lives, it is possible that they become habituated and are not influenced by the presence of humans at all (Snyder, 1975). Thirdly, visitors could also have an effect opposite to social enrichment, namely as a source of stress for the animals (Hediger, 1969; Hosey, 2000). Discovering which of these hypotheses holds the most truth is important, not only for the welfare of the animals but also to enhance behavioural studies conducted on zoo animals, and to improve the experience of zoo visitors (Hosey, 2000). The welfare of the animals is a particularly pressing issue when considering the visitor effect. If visitors truly cause stress this could have detrimental effects on the animals' physical and psychological health, with possible consequences ranging from an increase in aggressive behaviour to a decline in physical health and lower breeding success (Carlstead and Shepherdson, 1994)
To study stress in animals, it is important to note how stress expresses itself in behaviour. Broom and Johnson (1993) have identified some behavioural indicators of animal welfare, which can be divided into short-term and long-term indicators. Short-term indicators are decreased orientation and an increase in startling or reflex responses, while long-term indicators of poor welfare are an increase in aggression, apathy and stereotypic behaviour. The presence of stereotypic behaviour, defined as a repetition of certain movements that do not serve any apparent purpose, is commonly used as an indicator of stress and poor welfare (Boorer, 1972; Broom, 1991; Marriner and Drickamer, 1994). However, it is unclear if the relationship between stress and stereotypy is truly that simple (Mason, 1991). Individual animals can have very different responses to stressful situations, making a generalisation of certain stereotypic behaviour to an entire species more difficult (Carlstead, 1996).
Another complicating factor is the causality of visitor–animal interactions: visitors can affect animal behaviour, but how an animal behaves can also attract attention from visitors. While most evidence supports the visitor effect hypothesis, the hypothesis of visitor attraction has also been found to be true in some situations (Hosey, 2000; Margulis et al., 2003). Since the visitor effect and the visitor attraction hypotheses are not mutually exclusive, it can be difficult to differentiate which one led to the other. In some species, primates in particular, it has been found that the animals become more active when visitors are active and direct more behaviour at their audience (Hosey, 2000).
Primates stand out as the most researched taxon in the study of the visitor effect, while there has been very little focus on non-primate species (Margulis et al., 2003). Primates are expected to be more affected by the presence of visitors, since they are social animals, and therefore they often pay more attention to humans. An example of a taxon that is considered to experience very little effect from zoo visitors are felids, since they are less social and also large in body size. The size of the animal is considered a factor in the visitor effect, since it is hypothesised that small animals view visitors as threats, while larger animals would in a normal situation consider them to be prey so are less affected by their presence (Chamove et al., 1988; Davey, 2007; Margulis et al., 2003). However, it could be that solitary animals, such as felids, experience even more stress from visitors than social animals, since in natural circumstances they do not frequently encounter other animals or humans. Studies into the visitor effect in felids have found mixed results. High visitor densities were found to have a negative effect on pumas (Maia et al., 2012), leopards (Mallapur and Chellam, 2002) and jaguars (Sellinger and Ha, 2005; Vidal et al., 2016) but other studies found no effect for cheetahs (O'Donovan et al., 1993) and felids in general (Margulis et al., 2003). These contradictory results and a lack of research into some felid species specifically make this taxon an interesting one to research.
Some studies have been done on the effect of humans on felids in the wild. Siberian tigers (Panthera tigris altaica), also known as Amur tigers, live in the far east of Russia. They are nocturnal animals (Bashaw et al., 2007) and are considered to be cold-climate species, as they are more active during winter than summer and can withstand harsh temperatures (Langman et al., 2015). In the habitat of the Siberian tigers, human population density is very low. It was found that in areas where the roads are often used by people, the tigers spent less time at their kill sites and ate less of their kill (Kerley et al., 2002). This suggests that at least in the wild, these felids avoid human contact and are disturbed by it, something which could also hold true in captivity. In the case of Siberian tigers specifically, stressful situations in captivity are very undesirable, since a recent census conducted by the Russian government and WWF indicated that only an estimate of 530 individuals remain in the wild (Amirkhanov and Aramilev, 2015). Recently, human disturbances in the habitat of Siberian tigers have increased, and if this trend continues Siberian tigers could be severely threatened, as these felids need very large territories (Kerley et al., 2002). As a result, captive breeding programmes might play a crucial role in the survival of the species. For this reason, Siberian tigers were chosen as the species of study in this article.
Two Siberian tigers housed in an enclosure at Dierenpark Amersfoort, the Netherlands, were observed to investigate what effect visitor density has on their behaviour. Based on the human disturbance of wild Siberian tigers and the effect of visitors found in other felid species, a number of hypotheses can be formulated. Firstly, since tigers are nocturnally active, they are expected to show more activity during the Morning and Afternoon and the time of day could also affect their response to the visitors. Secondly, that the tigers show an increase in stress-related behaviours, such as stereotypy, when visitor densities increase. Thirdly, the type of stress-related behaviour may vary per individual, since stress can be expressed quite differently per animal. Fourthly, because the activity of Siberian tigers varies per season, average temperature is also hypothesised to affect their behaviour.
Material and methods
Subjects
The subjects of the case study were two Siberian tigers; Ilya, a 12-year-old male, and Angara, a 7-year-old female, housed together in an enclosure at Dierenpark Amersfoort, the Netherlands. Both tigers were born in captivity, respectively in Marwell Wildlife in the United Kingdom and Leipzig Zoo in Germany, and raised by their parents. Two years prior to observations, in June 2014, Angara had given birth to male triplets. The last cub was transferred to another zoo mid-August, approximately three weeks before observations started.
Housing and maintenance
The two tigers shared an outside enclosure, where they were on display during visitor hours from approximately 9:00 until 18:00 from April up to and including October and 10:00 until 17:00 during the rest of the year. At night they were kept in separate indoor enclosures of about 9 m2. The outside enclosure was 563 m2 and had no sub-compartments. Figure 1 shows a schematic map of the enclosure. The enclosure contained five spots where visitors could easily see the animals, marked with an X on the map (Figure 1). For this article, only visitors standing at the two large glass walls, the red and green Xs, were taken into account. This is because, firstly, most visitors gathered at these places; and secondly, since the glass was fully transparent and reached the floor, the tigers were expected to notice the visitors the most when they were standing at these spots. From the main viewing area (green X), most of the enclosure was visible to the public and there was no place in the enclosure where the animals were completely obscured from view.
The floor of the enclosure was a mix of earth and stones, with some slopes and mounds. In front of the main viewing area's glass, there was a stone platform and a pool filled with water. Fallen tree trunks were placed in various locations in the enclosure, such as over the pool and between two mounds. Vegetation was present in the form of bushes, trees and grass and there were several wooden platforms for the tigers to lie on. In the corner of the enclosure there was a small den and a hatch to an indoor enclosure. In both structures the tigers were still visible to the public. The indoor enclosure, used for feeding, had a concrete floor and wall and was 13 m2. It contained a hatch to another indoor enclosure as well as a steel fence through which the keepers could interact with and feed one of the tigers. There was another double glass wall at the side of this indoor enclosure, allowing visitors to look inside.
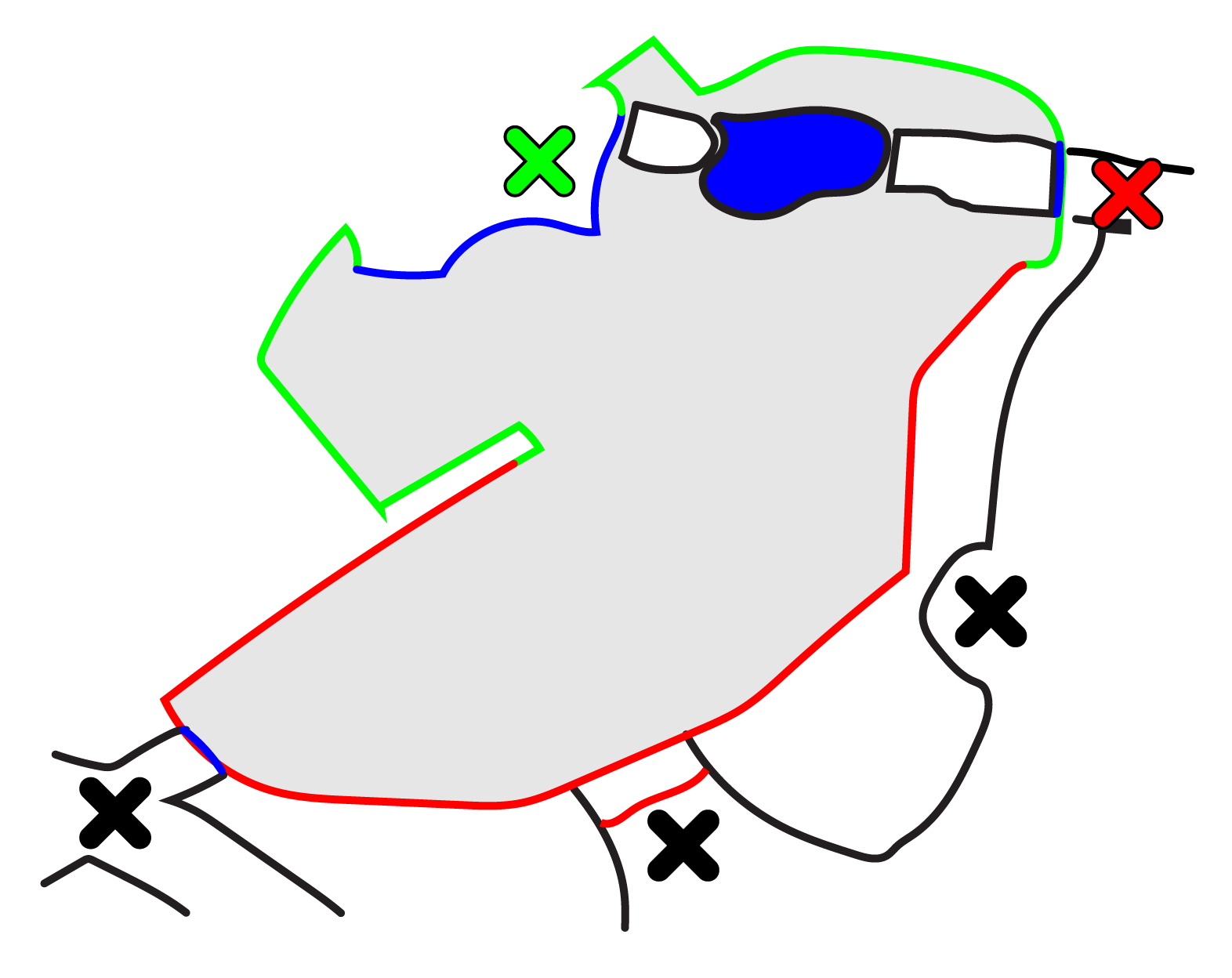
Figure 1: A schematic map of the tiger enclosure. Green lines indicate solid walls, red lines chain-link fences and blue lines floor-to-ceiling double glass walls. X marks viewing spots, the green X being the main viewing area and the red X the other glass wall. Filled out with blue is a pool.
The tigers were given enrichment on a weekly schedule (Table 1), ranging from object enrichment such as balls and fire hoses to olfactory enrichment in the form of sprayed perfumes and other scents. Feeding enrichment was also given to the animals by for instance hanging or burying meat.
| Day | Enrichment |
|---|---|
| Monday | Olfactory enrichment with spices |
| Tuesday | Object enrichment |
| Wednesday | Feeding enrichment |
| Thursday | Olfactory enrichment with perfume |
| Friday | Olfactory/object enrichment with manure |
| Saturday | Alternating object and food enrichment |
| Sunday |
Table 1: Enrichment schedule of the tigers
During feeding, which occurred around 12:30 on the days of observation, both tigers first went inside and were kept separately while the outdoor enclosure was cleaned, the pool refilled and food and enrichment were placed. Then one of the tigers was allowed to go outside to eat, while the other was fed in the small indoor enclosure. After both tigers were done eating, both were locked outside again. Usually, this entire process took between one and two hours. The tigers were fed daily, with the male receiving 5300 grams of meat per day and the female 2500 grams. The meat came from various animals: they received chicken, rabbit and lamb one day per week each, and beef or horse on the remaining four days.
Observations
Observations took place every Wednesday from 7 September 2016 until 23 November 2016. Focal sampling was used to collect the data and a wide range of behaviours was scored to capture the daily activity budget of the animals. The ethogram used (Appendix 1) was based on a standardised ethogram for felines developed by Stanton et al. (2015). A few changes were made to optimise the ethogram for this article: vocalisations were excluded, since these could not be heard through the glass, and yawning, which was only observed in a non-aggressive context, was classified as calm behaviour. In the absence of the opportunity to take cortisol samples and measure stress levels directly, certain behaviours such as stereotypy, apathy and increased aggression or decreased affiliation were used as indicators of a sub-optimal psychological and physical health.
The focal observations were divided into sessions of five minutes with 15-second intervals between sampling points. Point behaviours such as yawning were written down whenever they occurred during the session. Durational behaviours such as lying were scored when they were initiated and at every sample point. A change in durational behaviour, for instance from lying to walking, was only scored after the new behaviour had occurred for three consecutive seconds. After three sessions, so 15 minutes, the observer switched attention to the other animal. On each day, observations took place in three time slots (Table 2), which were used to divide the data based on time of day. A total of 18 consecutive observations took place in each time slot and there were no observations while the tigers were separated during feeding. Since the time and duration of feeding varied, the Morning and Midday time slots were made half an hour longer in order to encompass all of the observations. From the start of November onwards, the zoo adapted winter opening times, meaning it closed at 17:00 and the tigers were taken inside at 16:30. In order to be able to continue observations, the Midday and Afternoon time slots were shortened to 12 observations to maintain a proper balance of observations per animal. Whenever possible, 18 observations were still conducted in these time slots.
| Period of day | Time slot |
|---|---|
| Morning | 10:30–12:30 |
| Midday | 13:30–15:30 |
| Afternoon | 16:00–17:30 (Winter: 15:30–16:30) |
Table 2: Time slots of observation
The average visitor count per session was calculated by summing the amount of visitors present in front of both large glass walls before and after each five-minute session and dividing the obtained number by two. The observer was excluded from all visitor counts and if the average visitor count was not a whole number, it was rounded up.
This resulted in 288 observations, equal to 24 hours, per focal animal, as well as an average visitor count for each five-minute sampling period.
Analysis
To study influence of average visitor count on aggressive behaviours, affiliative behaviours, stereotypy and (in)activity, several measures were taken. All behaviours were classified according to their category in the ethogram (Appendix 1) and in order to quantify activity versus resting, a selection was made of behaviours that represent these states. In respect to the ethogram, all locomotive behaviours (e.g. walking, running), active behaviours (e.g. dragging, carrying) as well as playful behaviours and exploring, were considered to be active behaviours. All behaviours from the inactivity category (e.g. sleeping, lying) plus huddling comprised the resting behaviours. Using hourly weather records from the Royal Netherlands Meteorological Institute (KNMI), the average temperature in Celsius per time slot per day was calculated. Lastly, the average visitor count was divided into groups (Table 3), based on observations. If more than ten visitors were present, it was considered to be crowded, since at this point the glass walls would be entirely occupied by visitors.
| Visitor Group | Number of Visitors |
|---|---|
| Low | 0 to 5 |
| Medium | 6 to 10 |
| High | 11 or more |
Table 3: Division of visitor groups
The effect of average visitor count on the frequency of stress-associated behaviours was compared by non-parametric analysis, using Spearman's correlation tests and the Jonckheere-Terpstra (JT) test. All tests were conducted for the male and female tiger separately, to investigate whether there were differences in personality and therefore, stress response. SPSS version 24 was used for statistical analyses and the significance level α was set at .01 rather than .05, since not every observation could be considered an independent event.
Results
During the observational period, a total of 16 behaviours were not observed (marked with an asterisk in Appendix 1). Among these were all reproductive behaviours, therefore this category was excluded from analysis. Visitor-wise, the Midday time slot was the busiest period (mean number of visitors = 9.22, SD = 7.14, N = 192), followed by Morning (mean = 6.31, SD = 4.65, N = 210) and Afternoon (mean = 5.07, SD = 3.68, N = 174). The activity of the tigers was found to vary depending on the time of day (Figure 2). The female tiger was least active during the Midday period, while the male showed little difference in active behaviours between time periods, but did show more resting behaviours during the Morning. Because of the observed differences in behaviour depending on time of day, all analyses were conducted separately per time slot.
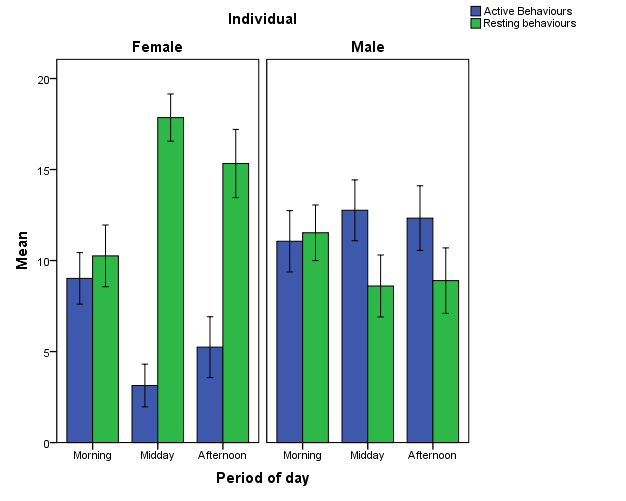
Figure 2: Mean of active and resting behaviours per individual depending on time of day.
From Figure 2 and an overview of their daily activity budget (Figure 3), it is noticeable that the male and female tiger varied considerably in their behaviour. While the male spent a large portion of the day locomoting, the female spent more time inactive and on maintenance behaviours, such as self-grooming. 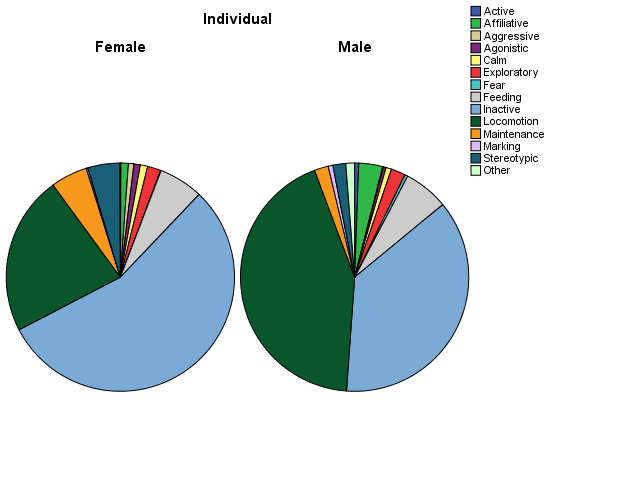
Figure 3: Pie charts displaying the daily activity budget per individual.
Female's behaviour With increased visitor count, active behaviours were hypothesised to decrease while resting behaviours would increase. For the female, increased visitor count correlated with decreased activity (Spearman's correlation, r = -0.39, p < .001; JT test, TJT = 1053, z = -3.17, p = .002) and increased resting behaviours (Spearman's correlation, r = 0.38, p < .001; JT test, TJT = 2044, z = 3.17, p = .002) during the Morning. During the Midday time slot, no significant correlation was found. In the Afternoon, resting behaviours increased significantly (Spearman's correlation, r = 0.30, p = .005; JT test, TJT = 1383, z = 2.84, p = .005), but a decrease in active behaviours was only significant on the alpha-level of .05 (Spearman's correlation, r = -0.26, p = .014; JT test, TJT = 815, z = -2.41, p = .016). These results are summarised in Figure 4 and all statistical results can be found in Appendix 2. 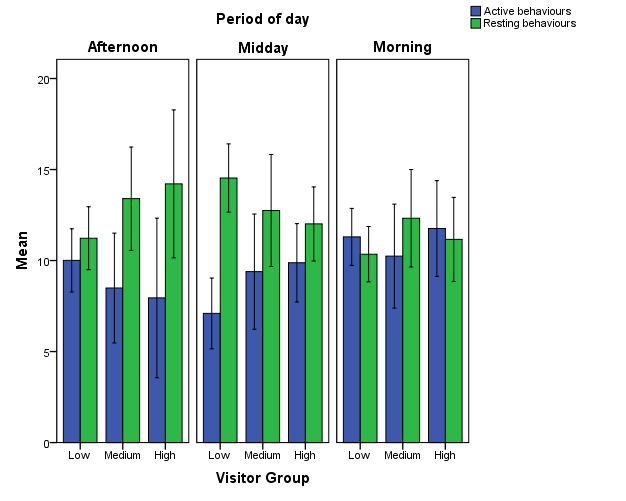
Figure 4: Mean of active and resting behaviours for the female tiger.
Throughout the observational period, the female tiger showed more aggressive behaviour than the male tiger (Figure 5). In the case of the female tiger, aggressive behaviour increased when visitor count increased; however, a significant increase in aggressive behaviour was only observed in the Morning time slot (Figure 6; Spearman's correlation, r = 0.37, p < .001; JT test, TJT = 1847, z = 3.40, p = .001). Affiliative behaviours were scarcely observed (N = 42) and showed no consistent patterns related to visitor count.
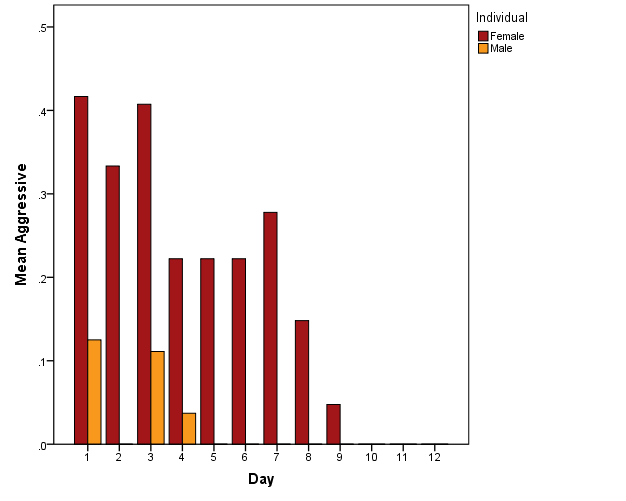
Figure 5: Mean of aggressive behaviour per individual per day of observation.
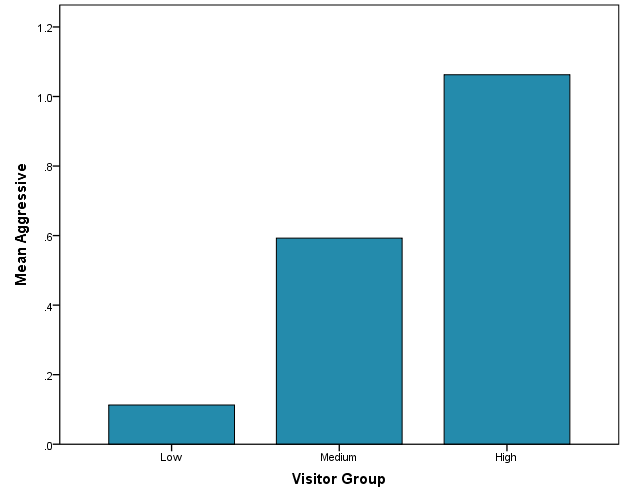
Figure 6: Mean frequency of aggressive behaviour per visitor group for the female tiger during the Morning time slot.
The female tiger showed little traditional stereotypic behaviour such as pacing and there was no significant increase in stereotypic behaviour during any of the time slots (see Appendix B).
Male's behaviour
The activity of the male tiger varied little depending on visitor count or time of day. However, in the Morning time slot an increase in active behaviours of the male tiger correlated with an increase in visitors (Spearman's correlation, r = 0.29, p = .002; JT test, TJT = 1988, z = 2.57, p = .010). There was no significant change in frequency of resting behaviours related to visitor count.
The male only very rarely displayed aggressive behaviours (N = 5), and the occurrence of aggressive behaviour did not show any link to visitor count. Similarly to for the female, no significant trend was found in the distribution of affiliative behaviour of the male tiger. However, it is of interest to note that the male initiated affiliative behaviour more frequently than the female tiger did during the observational period (Figure 7).
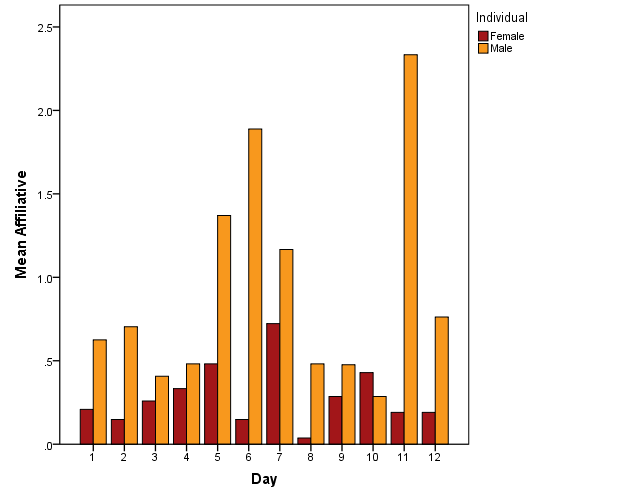
Figure 7: Mean of affiliative behaviour per individual per day of observation.
Contrary to the female, the male tiger engaged in more stereotypic behaviour, most frequently in the form of pacing. The frequency of stereotypic behaviour increased significantly with visitor count in the Morning (Spearman's correlation, r = 0.27, p = .006; JT test, TJT = 1800, z = 2.69, p = .007) and Midday time slot (Spearman's correlation, r = 0.30, p = .003; JT test, TJT = 1751, z = 2.60, p = .009). In the Afternoon, no significant increase in stereotypic behaviour with higher visitor density was observed (Figure 8).
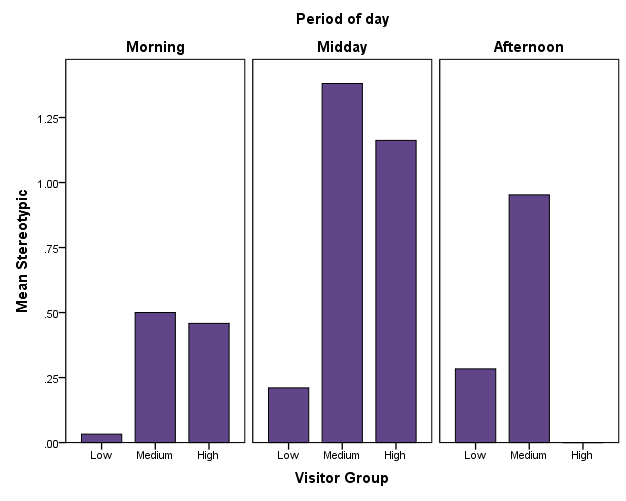
Figure 8: Mean of stereotypic behaviour for the male tiger depending on time of day.
Average temperature
The average temperature in Celsius varied widely on observation days (mean temperature = 14.8, min = 3.9, max = 30.1). An increase in temperature was significantly correlated with an increase in visitor count (Figure 9; Spearman's correlation, r = 0.437, p < .001).
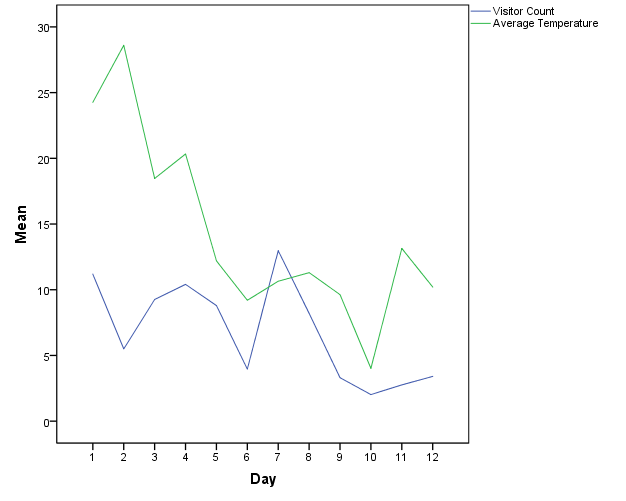
Figure 9: Average visitor count and temperature per day of observation.
Average temperature was also found to affect the behaviour of the tigers. In the case of the female, warmer weather was correlated to increased resting behaviours in all time slots (Spearman's correlation, r = 0.28, p < .001) and decreased active behaviours in the Morning (Spearman's correlation, r = -0.36, p < .001) and Afternoon (Spearman's correlation, r = -0.30, p = .005).
No influence of weather on the aggressive behaviours of the female was found.
For the male, weather seemed to have no influence on active and resting behaviours. Similarly to visitor count, an increase in average temperature was found to correlate with an increase in stereotypic behaviours in the male in the Morning (Spearman's correlation, r = 0.34, p < .001) and the Midday time slot (Spearman's correlation, r = 0.46, p < .001).
Discussion
Time of day
While differences in activity depending on time of day were found, the expected nocturnal pattern was not present in either of the tigers. The female tiger did rest significantly more in the Midday period; however, the tigers were fed prior to this time slot. Wild tigers have been observed to kill and mate whenever possible, including during the day, suggesting their nocturnal patterns are flexible (Bashaw et al., 2007). Being fed during daylight hours on a daily basis for a significant part of their lives could have caused the tigers to increase their daytime activity, especially considering that at night they were kept in smaller enclosures with little opportunity for active behaviours.
The varying duration and timing of feeding led to observations being cut short on some occasions. On 19 October, the Afternoon time slot did not take place, since the tigers were fed at 16:00 instead of 12:30. This feeding regime had only been implemented a month before observations started and it is possible the tigers were not yet accustomed to this change. In the old regime, the tigers were fed in the morning before the park opened. This could have influenced their behaviour in the Morning time slot.
Individual differences
The male and female tiger varied strongly in personality, and were also found to respond to high visitor density in different ways. This is in line with the findings of Carlstead (1996) and makes generalising the findings of any behavioural study in individuals to an entire species difficult. It is difficult to say if these differences were due to gender, since little research has been done on behavioural differences between male and female felines.
Aggression and affiliation
An increase in aggressive behaviours is seen as a possible consequence of repeated exposure to stress (Carlstead and Shepherdson, 1994). The female showed an increase in aggressive behaviour with higher visitor count in all time slots, although only significant in the Morning time slot. Overall, the female displayed more aggression than the male, although often these were short, isolated incidents. The following situation was observed regularly: the male tiger would approach the female, often attempting affiliative behaviour such as head rubbing. However, the female would prevent or cut short the affiliation by threatening or sometimes even physically attacking the male. The attack was usually limited to a strike with her paw or lunging at the male, who would then retreat nearly instantly.
The female's high display of aggression could be due to various factors. After giving birth to triplets, she was put on anticonception, which was hypothesised by the zoo staff to cause her to become more aggressive towards the male. However, there is little research on the effect of anticonception on felids and more information would be required in order to investigate this further. The removal of her cubs could also have been stressful for the female, as the last cub left only three weeks before observations started. Nonetheless, while these factors could perhaps account for the high incidence of aggressive behaviours by the female, it cannot explain the observed link to visitor count. Therefore, it is also possible that for the female, aggressive behaviour towards the male provided a method of redirecting stress or discomfort caused by a large number of visitors, perhaps stemming from an inability to escape from view (Vidal et al., 2016). The redirection of aggression towards uninvolved third parties has been found in many species of primates (Arnold and Whiten, 2001), but has scarcely been researched in felids. Since the aggressive actions were short and not easily noticeable unless paying close attention to the female, it is unlikely that the visitor attraction hypothesis could be true in this situation.
For both tigers, no pattern was found regarding affiliative behaviour and visitor count.
Stereotypy
Only the male tiger was found to display stereotypic behaviour when visitor count increased in the Morning and Midday time slots. Stereotypy can be influenced by other factors as well, such as size and complexity of the enclosure (Lyons et al., 1997) and the absence of enrichment (Swaisgood and Shepherdson, 2005). The enclosure, while quite complex, was 563 m2 large, which is only a fraction of the territory of a wild Siberian tiger. A single female Siberian tiger requires around 450 km2, a single male even more (Goodrich et al., 2010). However, enrichment was present on all observation days, which has been shown to reduce stereotypic behaviour in felids (Skibiel et al., 2007; Swaisgood and Shepherdson, 2005; Szokalski, et al., 2012). The feeding regime could also have affected stereotypy, since animals often display stereotypic behaviour in anticipation of food (Carlstead, 1998; Watters, 2014). Lastly, since pacing is difficult to quantify and differentiate from walking and other active behaviours, it is possible that patrolling and marking territory was misinterpreted as pacing.
Activity and resting
One of the hallmarks of poor welfare as described by Broom and Johnson (1993) is an increase in inactivity, which could be seen as either a decrease in active behaviours, or an increase in resting behaviours. The female showed significantly less active and more resting behaviours in the Morning and Afternoon time slots. For the male, contrary to expectations, an increase in active behaviours correlated with high visitor density in the Morning time slot. This increase in activity could be related to the aforementioned point of the difficulty in distinguishing walking from pacing.
Temperature
The influence of average temperature and visitor count on behaviours are difficult to separate. When temperatures increase, people are more likely to go to the zoo and cold-climate animals such as Siberian tigers become less active. In the case of the female tiger, both visitor count and temperature were found to have the same effect on active and resting behaviours. For the male, high temperatures and visitor count increased the amount of stereotypic behaviour in the Morning and Midday. While in some cases, changes in stress behaviours could have been caused by a higher visitor density, this is near impossible to distinguish from temperature influences. The male's active and resting behaviours were unaffected by the temperature, which can be explained by the fact that he made frequent use of the pool, while the female only did so on rare occasions.
The only behaviour that was exclusively influenced by visitor count and showed no relation to temperature was the amount of aggressive behaviour displayed by the female.
Limitations
As this article was a case study conducted in a limited period of time, some limitations arose that should be taken into account for future research. For instance, most visitors stay at an exhibit for less than five minutes; therefore, the obtained visitor counts may not have been accurate representations of the number of visitors present.
Additionally, the JT test used for analysis makes use of the visitor groups, in this article the division of visitors was made based on substantive grounds. However, it could be that the tigers experience this differently, and therefore another division would have been more suitable. The visitor groups were also not equally distributed over the time slots, leading to skewed results per time of day. For example, in the Afternoon time slot there were almost no occurrences of a high visitor group, which decreases the statistical chance of finding a correlation.
The strong relationship of temperature to visitor count implies that in future studies, measures should be taken to separate these variables. Only then it can be said with more certainty if for instance the resting behaviours of felids are influenced mostly by weather, or by the amount of visitors present.
Conclusion
In summary, contrary to Margulis et al. (2003) but similarly to the results found by Maia et al. (2012), Mallapur and Chellam (2002), Sellinger and Ha (2005), and Vidal et al. (2016), high visitor densities were found to affect the behaviour of the two captive tigers in this case study. However, only in the case of the female tiger can it be concluded that the stress behaviour, an increase in aggression, was affected by visitor count alone and not by other closely related variables such as temperature. An important factor is that the tigers were always visible to the public. Creating a location where the tigers can be obscured from the view of visitors might decrease stress-related behaviours. The changes in the behaviour of the tigers caused by visitors are small and hard to notice, therefore easy to overlook. However, the fact that they are not as noticeable as in other species such as primates, does not mean that stress behaviours in tigers are absent. While this article is a descriptive case study, it does suggest that felids such as Siberian tigers are more sensitive to the influence of visitors than is usually thought.
Acknowledgements
I thank Raymond van der Meer and Dierenpark Amersfoort for allowing observations to take place; Marc Belt for providing information about the tigers; Dr E. H. M. Sterck, Dr I. A. Flameling, Dr M. J. H. M. Duchateau and Michael Edelijn for their supervision; and Jacqueline Tempel for her assistance with the statistical analyses.
List of illustrations
Figure 1: Schematic map of the tiger enclosure.
Figure 2: Graph of mean active and resting behaviours per individual depending on time of day.
Figure 3: Pie charts of daily activity budget per individual.
Figure 4: Mean of active and resting behaviours for the female tiger.
Figure 5: Mean of aggressive behaviour per individual per day of observation.
Figure 6: Mean frequency of aggressive behaviour per visitor group for the female tiger during the Morning time slot.
Figure 7: Mean of affiliative behaviour per individual per day of observation.
Figure 8: Mean of stereotypic behaviour for the male tiger depending on time of day.
Figure 9: Graph of average visitor count and temperature per day of observation.
List of tables
Table 1: Enrichment schedule of the tigers.
Table 2: Time slots of observation.
Table 3: Division of visitor groups.
Appendix 1
Ethogram Panthera tigris. An asterisk marks unobserved behaviour.
| Behaviour | Description |
|---|---|
| Active | |
| Carry | Cat picks (modifier) up off the ground and moves it to another location. |
| Drag | Cat moves (modifier) from one location to another without picking it up off the ground. |
| Wrestle* | Cat engages in physical contact with (modifier), whereby the cat struggles with (modifier). Can include pulling (modifier) toward itself with its forelegs and perform raking movements with the hind legs. |
| Affiliative | |
| Huddling | Cat is at rest, lying or sitting with body in contact with (modifier). |
| Social play | Cat interacts with other cat in a non-serious manner (i.e. where there is no intention to harm). |
| Object play | Cat interacts with object in a non-serious manner (i.e. where there is no intention to harm). |
| Allogroom | Cat licks the fur of another cat's head or body. |
| Social roll* | While lying on the ground, cat rotates body from one side to another. During the roll, the back is rubbed against the ground, the belly is exposed and all paws are in the air. Cat may continue rolling from side to side. |
| Head rub | Cat rubs its head against (other cat). |
| Head rub Object | Cat rubs its head against an object. |
| Social sniff | Cat smells (modifier) by inhaling air through the nose. |
| Aggressive | |
| Attack | Cat launches itself at (modifier) with extended forelegs and attempts to engage in physical combat. |
| Bite* | Cat snaps teeth at and is successful in biting (modifier). |
| Charge | Cat rushes forward (modifier). |
| Chase* | Cat runs rapidly in pursuit of (other cat). |
| Cuff | Cat strikes at (modifier) with forepaw and contact is made. Claws are usually extended. |
| Fight | Cat engages in physical combat with another cat. |
| Rear* | Cat stands up on its hind legs with forelegs toward or against (modifier) |
| Strike at | Cat swipes forepaw at (modifier) but no contact is made. |
| Threaten | Cat directs aggressive behaviour toward (modifier) without making any physical contact with it. |
| Agonistic | |
| Approach | Cat moves toward (modifier) while looking at it. |
| Social stare | Cat gazes fixedly at (modifier) and is not easily distracted. In the case of social stare, gaze may be directed at another cat's eyes. |
| Calm | |
| Kneading* | Cat pushes forepaws into the ground or (modifier) in a rhythmic, kneading motion. |
| Scratching | Cat scratches its body using the claws of its hind feet. |
| Stretching | Cat extends its forelegs while curving its back inwards. |
| Yawn | Cat opens its mouth widely while inhaling, then closes mouth while exhaling deeply. |
| Exploratory | |
| Explore | Cat moves around attentively while sniffing the ground and/or objects. |
| Investigate | Cat shows attention toward a specific stimulus by sniffing and/or pawing at it. |
| Paw | Cat pats (modifier) with its forepaw(s). Claws are usually retracted. |
| Sniff (any) | Cat smells (modifier) by inhaling air through the nose. |
| Fear | |
| Avoid | Cat moves, or changes direction while moving, in order to keep away from (modifier). |
| Crouch | Cat is alert and positions the body close to the ground, whereby all four legs are bent, and the belly is touching (or raised slightly off of) the ground. |
| Flee | Cat runs away from (modifier). |
| Flinch | Cat approaches and/or sniffs (modifier), but abruptly stops and retreats or flees from (modifier). |
| Head shake | Cat rotates head from side to side. |
| Feeding | |
| Drink | Cat ingests water (or other liquids) by lapping up with the tongue. |
| Eat | Cat ingests food (or other edible substances) by means of chewing with the teeth and swallowing. |
| Lick object | Cat's tongue protrudes from mouth and strokes (food). |
| Pounce | Cat leaps onto (food). |
| Stalk | Slow, forward locomotion in a crouched position directed towards (modifier), with head kept low and eyes focused on (modifier). |
| Inactive | |
| Lying | Cat's body is on the ground in a horizontal position, including on its side, back, belly, or curled in a circular formation. |
| Sitting | Cat is in an upright position, with the hind legs flexed and resting on the ground, while front legs are extended and straight. |
| Sleeping | Cat is lying on the ground with its head down and eyes closed, performing minimal head or leg movement and is not easily disturbed (defined as lying without movement and eyes closed for 10 tallies). |
| Standing | Cat is in an upright position and immobile, with all four paws on the ground and legs extended, supporting the body. |
| Locomotion | |
| Climb | Cat ascends and/or descends an object or structure. |
| Follow | One cat travels closely behind (modifier). |
| Jumping | Cat leaps from one point to another, either vertically or horizontally. |
| Running | Forward locomotion in a rapid gait, which is faster than walking or trotting. |
| Trotting | Forward locomotion at a swift gait performed with alternating steps. Movement is faster than walking but slower than running. |
| Walking | Forward locomotion at a slow gait. |
| Maintenance | |
| Defecate | Cat releases faeces on the ground while in a squatting position. |
| Groom | Cat cleans itself by licking, scratching, biting or chewing the fur on its body. May also include the licking of a front paw and wiping it over one's head. |
| Lick self | Cat licks itself once. |
| Urinate | Cat releases urine on the ground while in a squatting position. |
| Marking | |
| Clawing | Cat drags front claws along an object or surface, likely leaving visual marks behind. |
| Hind feet scraping | Cat scrapes hind feet on the ground in a backwards direction, shuffling one foot after the other. |
| Solitary roll | While lying on the ground, cat rotates body from one side to another. During the roll, the back is rubbed against the ground, the belly is exposed and all paws are in the air. Cat may continue rolling repeatedly from side to side. |
| Urine spray | While standing with the tail raised vertically, cat releases a jet of urine backwards against a vertical surface or object. The tail may quiver as urine is discharged. |
| Reproductive | |
| Copulation* | Male mounts female and intromission is achieved. |
| Flirting run* | Female cat feigns running away from a breeding partner. |
| Intromission* | The male's penis enters the female's vagina. |
| Lordosis* | Female cat raises hindquarters while lowering forequarters to the ground, presenting genitals to male, tail often averted to one side. The position is sometimes accompanied by treading of the hind legs. |
| Mount* | A male cat attempts intromission by straddling over the female with front and hind feet. In small cats this may be accompanied by a nape bite and/or treading movements of the hind feet. |
| Nape bite* | The male performs an inhibited nape bite, where he will place his mouth on or around the back of the female's neck at the moment of, or just after, ejaculation, but is unlikely to actually bite down. |
| Pelvic thrust* | While in a mounted position, the male makes searching and/or thrusting movements with his pelvic region against the anogenital region of the female. |
| Stereotypic | |
| Fur-plucking | Cat excessively grooms a specific area of its body. This can include any tail- and paw-sucking actions. May result in removal and visible loss of fur, as well as skin irritation. |
| Head-rolling* | Cat tosses its head in a circular motion. |
| Pacing | Repetitive locomotion in a fixed pattern, such as back and forth along the same route. Can include walking, trotting and running. Movement seems to have no apparent goal or function. Must be performed at least two times in succession before qualifying as stereotypic. |
| Self-biting* | Cat bites or chews on an area of its own body, which may result in damage to the fur or skin. |
| Self-mutilation* | Cat performs any self-injurious behaviour (including self-biting and fur-plucking), which may result in a visible hair loss and skin irritation or abrasion. |
| Other | |
| Pool | Cat is in or interacting with pond (swimming etc.), except for drinking from it. |
| Not visible | Cat is not visible to the observer. |
Appendix 2
Overview of all obtained statistical results, behaviours are bolded if the results are significant at the .01 level.
Female Tiger:
| Behaviours | Spearman's Correlation Test | Jonckheere-Terpstra Test |
|---|---|---|
| Morning | ||
| Active | r = -0.39, p < .001 | TJT = 1053, z = -3.165, p = .002 |
| Resting | r = 0.38, p < .001 | TJT = 2044, z = 3.165, p = .002 |
| Aggressive | r = 0.37, p < .001 | TJT = 1847, z = 3.404, p = .001 |
| Affiliative | r = 0.14, p = .170 | TJT = 1623, z = 0.701, p = .483 |
| Stereotypic | r = -0.11, p = .270 | TJT = 1438, z = -0.824, p = .410 |
| Midday | ||
| Active | r = 0.20, p = .056 | TJT = 1748, z = 2.316, p = .21 |
| Resting | r = -0.16, p = .112 | TJT = 1184, z = -2.009, p = .045 |
| Aggressive | r = 0.16, p = .118 | TJT = 1566, z = 1.652, p = .099 |
| Affiliative | r = 0.10, p = .315 | TJT = 1583.5, z = 1.505, p = .132 |
| Stereotypic | r = 0.20, p = .044 | TJT = 1601, z = 1.947, p = .052 |
| Afternoon | ||
| Active | r = -0.26, p = .014 | TJT = 815, z = -2.408, p = .016 |
| Resting | r = 0.30, p = .005 | TJT = 1383, z = 2.838, p = .005 |
| Aggressive | r = 0.06, p = .598 | TJT = 1082.5, z = 0.517, p = .605 |
| Affiliative | r = 0.26, p = .017 | TJT = 940, z = -1.725, p = .085 |
| Stereotypic | r = -0.18, p = .095 | TJT = 862.5, z = -2.377, p = .017 |
Male Tiger:
| Behaviours | Spearman's Correlation Test | Jonckheere-Terpstra Test |
| Morning | ||
| Active | r = 0.29, p = .002 | TJT = 1988 , z = 2.567, p = .010 |
| Resting | r = -0.21, p = .032 | TJT = 1309, z = -1.717, p = .086 |
| Aggressive | r = 0.15, p = .136 | TJT = 1659, z = 1.671, p = .095 |
| Affiliative | r = -0.02, p = .816 | TJT = 1477.5, z = -0.770, p = .441 |
| Stereotypic | r = 0.27, p = .006 | TJT = 1800.5, z = 2.688, p = .007 |
| Midday | ||
| Active | r = -0.11, p = .272 | TJT = 1300, z = -1.301, p = .193 |
| Resting | r = 0.000, p = .997 | TJT = 1505.5, z = 0.103, p = .918 |
| Aggressive | r = 0.17, p = .096 | TJT = 1520, z = 1.141, p = .254 |
| Affiliative | r = -0.13, p = .226 | TJT = 1403, z = -0.772, p = .440 |
| Stereotypic | r = 0.30, p = .003 | TJT = 1751, z = 2.603, p = .009 |
| Afternoon | ||
| Active | r = 0.06, p = .577 | TJT = 1016.5, z = 1.306, p = .191 |
| Resting | r = -0.01, p = .955 | TJT = 779.5, z = -0.855, p = .393 |
| Aggressive | r = 0.18, p = .092 | TJT = 913.5, z = 1.989, p = .047 |
| Affiliative | r = -0.01, p = .960 | TJT = 858, z = -0.181, p = .856 |
| Stereotypic | r = 0.11, p = .293 | TJT = 959, z = 1.301, p = .193 |
Notes
[1] Zoe Goldsborough is currently a Master's Student of Environmental Biology at Utrecht University.
References
Amirkhanov, A. M. and V. V. Aramilev (2015), 'The Amur tiger census in Russia 2014-2015', available at https://www.wwf.ru/data/amur/leaflet-tiger-2015-census-with-flag-interactive.pdf, accessed 30 September 2016
Arnold, K. and A. Whiten (2001), 'Post-conflict behaviour of wild chimpanzees (Pan Troglodytes Schweinfurthii) in the Budongo Forest, Uganda', Behaviour, 138 (5), 649–90
Bashaw, M. J., A. S. Kelling, M. A. Bloomsmith and T. L. Maple (2007), 'Environmental effects on the behavior of zoo-housed lions and tigers, with a case study of the effects of a visual barrier on pacing', Journal of Applied Animal Welfare Science, 10 (2), 95–109
Boorer, M. K. (1972), 'Some aspects of stereotyped patterns of movement exhibited by zoo animals', International Zoo Yearbook, 12, 164–68
Broom, D. M. (1991), 'Assessing welfare and suffering', Behavioural Processes, 25 (2–3), 117–23
Broom, D. M. and K. G. Johnson (1993), Stress and Animal Welfare, Dordrecht: Springer
Carlstead, K. (1998), 'Determining the causes of stereotypic behaviors in zoo carnivores. Toward appropriate enrichment strategies', in Shepherdson, D. J. (ed.), Second nature: Environmental enrichment for captive animals, Washington D.C., Smithsonian Institution Press, pp. 172–83
Carlstead, K. and D. J. Shepherdson (1994), 'Effects of environmental enrichment on reproduction', Zoo Biology, 13, 447–58
Chamove, A. S. and E. M. Moodie (1990), 'Are alarming events good for captive monkeys?', Applied Animal Behaviour Science, 27 (1–2), 169–76
Davey, G. (2007), 'Visitors' effects on the welfare of animals in the zoo: a review', Journal of Applied Animal Welfare Science, 10 (2), 169–83
Fernandez, E. J., M. A. Tamborski, S. R. Pickens and W. Timberlake (2009), 'Animal-visitor interactions in the modern zoo: Conflicts and interventions', Applied Animal Behaviour Science, 120 (1–2), 1–8
Goodrich, J. M., D. G. Miquelle, E. N. Smirnov, L .L Kerley, H. B. Quigley and M. G. Hornocker (2010), 'Spatial structure of Amur (Siberian) tigers (Panthera tigris altaica) on Sikhote-Alin Biosphere Zapovednik, Russia', Journal of Mammalogy, 91 (3), 737–48
Hediger, H. (1969), Man and Animal in the Zoo, London: Routledge and Kegan Paul
Hosey, G. R. (2000), 'Zoo animals and their human audiences: What is the visitors' effect?', Animal Welfare, 9 (2), 343–57
Kerley, L. L., J. M. Goodrich, D. G. Miquelle, E. N. Smirnov, H. B. Quigley and M. G. M. G. Hornocker (2002), 'Effects of roads and human disturbance on Amur tigers', Conservation Biology, 16 (1), 97–108
Langman, V. A., S. L. Langman and N. Ellifrit (2015), 'Seasonal acclimatization determined by non-invasive measurements of coat insulation', Zoo Biology, 34 (4), 368–73
Lyons, J., R. J. Young and J. M. Deag (1997), 'The effects of physical characteristics of the environment and feeding regime on the behavior of captive felids', Zoo Biology, 16, 71–83
Maia, C. M., G. L. Volpato and E. F. Santos (2012), 'A case study: The effect of visitors on two captive pumas with respect to the time of the day', Journal of Applied Animal Welfare Science, 15 (3), 222–35
Mallapur, A. and R. Chellam (2002), 'Environmental influences on stereotypy and the activity budget of Indian leopards (Panthera pardus) in four zoos in Southern India', Zoo Biology, 21 (6), 585–95
Margulis, S. W., C. Hoyos and M. Anderson (2003), 'Effect of felid activity on zoo visitor interest', Zoo Biology, 22 (6), 587–99
Marriner, L. M. and L. C. Drickamer (1994), 'Factors influencing stereotyped behavior of primates in a zoo', Zoo Biology, 13 (3), 267–75
Mason, G. J. (1991), 'Stereotypies: a critical review', Animal Behaviour, 41 (6), 1015–37
Morris, D. (1964), 'The response of animals to a restricted environment', Symposia of the Zoological Society of London, 13, 99–120
O'Donovan, D., J. E. Hindle, S. McKeown and S. O. Donovan (1993), 'Effect of visitors on the behavior of female cheetahs and cubs', Internation Zoo Yearbook, 32, 238–44
Sellinger, R. L. and J. C. Ha (2005), 'The effects of visitor density and intensity on the behavior of two captive jaguars (Panthera onca)', Journal of Applied Animal Welfare Science, 8 (4), 233–44
Skibiel, A. L., H. S. Trevino and K. Naugher (2007), 'Comparison of several types of enrichment for captive felids', Zoo Biology, 26 (5), 371–81
Snyder, R. L. (1975), 'Behavioral stress in captive animals', Research in Zoos and Aquariums, 41–76
Stanton, L. A., M. S. Sullivan and J. M. Fazio (2015), 'A standardized ethogram for the felidae: A tool for behavioral researchers', Applied Animal Behaviour Science, 173, 3–16
Swaisgood, R. R. and D. J. Shepherdson (2005), 'Scientific approaches to enrichment and stereotypies in zoo animals: What's been done and where should we go next?', Zoo Biology, 24 (6), 499–518
Szokalski, M. S., C. A. Litchfield and W. K. Foster (2012), 'Enrichment for captive tigers (Panthera tigris): Current knowledge and future directions', Applied Animal Behaviour Science, 139 (1–2), 1–9
Vidal, L. S., F. R. Guilherme, V. F. Silva, M. C. S. R. Faccio, M. M. Martins and D. C. Briani (2016), 'The effect of visitor number and spice provisioning in pacing expression by jaguars evaluated through a case study', Brazilian Journal of Biology, 76 (2007), 506–10
Watters, J. V. (2014), 'Searching for behavioral indicators of welfare in zoos: Uncovering anticipatory behavior', Zoo Biology, 33 (4), 251–56
To cite this paper please use the following details: Goldsborough, Z. (2017), 'The Effect of Visitor Density on the Behaviour of Two Siberian Tigers (Panthera tigris altaica) Housed in a Zoo Enclosure: A Case Study', Reinvention: an International Journal of Undergraduate Research, Volume 10 Issue 2: Featuring the Eramus+ BLASTER Project, https://warwick.ac.uk/fac/cross_fac/iatl/reinvention/issues/volume10issue2/blaster/goldsborough. Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal at warwick dot ac dot uk.