Assessment of Diet, Activity Budget and Daily Path-length of Sri Lanka's Endemic Western Purple-faced Leaf Monkey (Trachypithecus vetulus nestor) in a Human Modified Environment
by Richard Stephen Moore, Department of Anthropology, Oxford Brookes University [1]
ABSTRACT
During the course of a ten-week period, various studies were carried out on two troops of Sri Lanka's endemic western purple-faced leaf monkey (Trachypithecus vetulus nestor, hereinafter T. v. nestor), which is now recognised as Critically Endangered, and is included on a list of the world's Top 25 Most Endangered Primates. The aims were to increase the currently limited amount of information available on the T. v. nestor, in particular focusing on survival potential and adaptation possibilities to its decreasing forest habitat.
Activity budgets revealed that resting was the primary activity undertaken by T. v. nestor over the period of observation. Observations of feeding habits showed that the fruit and leaves of Artocarpus heterophyllus provided the main source of food for both troops. Substrate use was found to include a range of man-made substrates (including roof tops, fences and power lines). Measurement of daily path-lengths revealed slight variations between the two troops monitored.
Generally the results were consistent with other recent studies on T. vetulus species and colobine monkeys, suggesting that although the primates are showing adaptations to severe forest fragmentation and human-modified environments, there are still areas for concern. For example, dangers when crossing between fragments, increasing human-primate conflicts and the risk of permanent genetic isolation could all seriously affect the survival potential of this species.
KEYWORDS: Primate conservation, ecology, behaviour, locomotion, biodiversity, forest fragmentation.
INTRODUCTION
Sri Lanka has 89 species of mammal and 15 (17%) of these are endemic (Mittermeier et al., 1999). Five species of the endemic mammals are primates, and within these five, 12 sub-species are currently recognised (Parker, 2007). All of the primates of Sri Lanka are endangered, but of these the Horton Plains slender loris (Loris tardigradus nycticeboides) and the western purple-faced leaf monkey (Trachypithecus vetulus nestor) are Critically Endangered and have been placed on a list as one of the world’s Top 25 Most Endangered Primates (IUCN, 2001; Mittermeier et al., 2005; Parker, 2007; Nekaris and de Silva Wijeyeratne, 2008). The main threat to these primates is continuing deforestation, leading to direct habitat loss and problems associated with fragmentation (Laurance et al., 2001; Rudran, 2007;Nekaris and de Silva Wijeyeratne,2008).
THE STUDY SPECIES
The species T. vetulus belongs to the family Cercopithecidae and the sub-family Colobinae (Rowe, 1996). Within T. vetulus species four sub-species are currently recognised, and T. v. nestor is one of them (Groves, 2001; Dela, 2004). Trachypithecus v. nestor, like two of the other four sub-species, has clearly defined ranges within Sri Lanka’s Wet Zone (Dela, 2007). It ranges from north of Kalu Ganga River to the rain forests just south of Kurunegala (Groves, 2001; Dela, 2004; Nekaris and de Silva Wijeyeratne, 2008). Range size and daily path-lengths of T. vetulus are known to vary, and are usually affected by availability of food (Strier, 2003). However, the range sizes are comparatively small when compared to other leaf-monkeys (Roonwal and Mohnot, 1977). Colobines are aptly named the ‘leaf-eating monkeys’ owing to the nature of their specialised ‘leafy’ diet, and they can be found throughout Africa and Asia, in a broad range of habitat types (Kirkpatrick, 2007). A large complex ‘sacculated’ stomach enables them to process the cellulose present in leaves (Fleagle, 1999; Nekaris and de Silva Wijeyeratne,2008). Trachypithecus vetulus species, like most other colobines, are very successful tree dwellers and are highly adapted to their arboreal environment. Paradoxically, this level of specialist adaptivity, which made them so successful in the past, is also perhaps what makes them so vulnerable to recent and ongoing changes in their habitat (Davies and Oates, 1994).Trachypithecus v. nestor is endemic to the Wet Zone of Sri Lanka, and this area is presently experiencing severe deforestation and fragmentation of forests (Rudran, 2007; Dela, 2007; Parker, 2007). In fact, since the 1930s, 90% of its known range has now become built up with houses, temples, gardens and other areas of human activity, which is probably the main reason for the 80% decline in the population of primates over the last three generations (IUCN, 2001)
Recent studies of the habitat and ecology of T. v. nestor show that it appears to be subsisting mainly on mature fruit from gardens with only a small percentage of the plants consumed being wild native ones (Rudran, 2007; Dela, 2007, Nekaris and de Silva Wijeyeratne, 2008). In the past, however, a study on T. v. philbricki conducted by Hladik (1977) revealed that shoots and leaves amounted to 60% of its diet, flowers and buds were 12%, and fruit only amounted to 28%. This change in diet is probably due to a reduction of available wild foods rather than a preference to cultivated foods. It is the first time any colobine monkey population has been seen to adapt to a human cultivated fruit diet of this kind (Dela and Rowe, 2006). The long-term effects of this change in diet are not yet known, but they are likely to be detrimental, as T. v. nestor has a highly specialised stomach adapted to digesting mature leaves by using symbiotic bacteria present in the gut. Therefore changes in carbohydrate and sugar intake could seriously affect the gut fauna, affecting the ability to absorb adequate nutrients (Fleagle, 1999; Rudran, 2007). Another direct effect habitat loss has on T. v. nestor is that it often forces the primates to travel terrestrially due to lack of canopy continuity between fragments within its range. This puts it at risk of capture (for house pets), killing by dogs or speeding vehicles, electrocution while climbing up power lines and being shot while feeding in gardens (Dela, 2004; Rudran, 2007; Nekaris and de Silva Wijeyeratne, 2008).
The current study focuses on systematic observations of T. v. nestor in two small areas between the Talangama and Hokandara suburbs of Colombo, where much of this fragmentation of habitat is obvious. Many specific survival and adaptation problems described in the literature are evident here and this article will investigate certain behavioural and ecological characteristics with specific reference (i) diet; (ii) time budget of behavioural activities according to time of the day; (iii) and daily path-lengths of two free-living groups of T. v. nestor.
METHODS
The study site
The study was conducted from 18 May to 30 June 2007 in an area around the village of Hokandara South, situated close to the Talangama wet lands, and 11.3 kilometres south-east of Colombo, Sri Lanka (Figure 1).It is located at 6º 54’ North and 70º 55’ East and is part of the Kaduwella District of Colombo (Philip, 1991). The average annual temperature of the area is 27º Celsius and the average rainfall is approximately 2,500 mm (FRGI, 2004).
Fig. 1: Image of Sri Lanka depicting the study site taken from Google Earth.
Behavioural observations
The particular study area in the Wet Lands was chosen for research, as troops of T. v nestor in this region have until now not been extensively studied. During the study two bordering troops were followed, (troop A and B). Troop A was initially discovered through interviews with local residents who informed the researchers of their location within Hokandara-South village, and the neighbouring troop B was discovered during observations of troop A (Figure 2). In order to habituate the primates in both troops, allowing closer observations and familiarisation of the home ranges, short pilot studies were undertaken. The pilot studies lasted 5 days for troop A and 3 days for troop B. Hokandara village is characterised by small patchy areas of trees and corridors of trees usually defining house boundaries. The area is currently being developed with a number of newly built properties and many more houses under construction. Paddy fields on the north and east side of the village and a large tank (lake) to the west act as natural constrictive boundaries for the monkey troops.
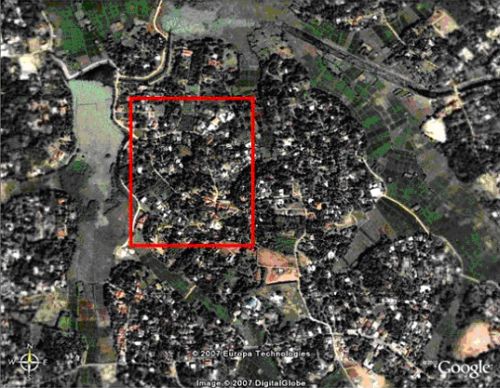
Fig. 2: Hokandara South - The red rectangle depicts the area of study containing the two troops studied. This image is taken from Google Earth (Scale of overall image = approximately 1,400 x 1,100m)
Individuals were counted and placed into sex and age categories. However, as there was often dense foliage in the tall trees in which the primates would rest/feed, and due to the timid nature of the animals, it was not possible to confidently identify all individuals separately, apart from a few with very distinguishable traits. For example, the dominant male of the group could be identified by his much larger size and his frequent vocalisations or “Whoop calls”. Troop compositions can be observed in Table 1.
|
Troop
|
Total Size
|
Adult Males |
Adult Females |
Sub-adults/ Juveniles |
Infants/ Newborns |
|
A |
19 |
1 |
8 |
5 |
5 |
|
B |
16 |
2 |
7 |
4 |
3 |
Table 1: Troop Composition for troops A and B
A variation of Patterson’s scan sampling method (2001) used by Dela (2007) was conducted at 15-minute intervals over a period of three days for each on the individuals that were visible at the time. This procedure enabled an estimate of activity/time budgets for various behaviours, which were calculated as a percentage over a set period of time, in addition to a percentage of different food types that were being consumed during a daily period. The types of behavioural categories used can be observed in Table 1.
|
Activity |
Description |
|
Moving |
Locomotion including walking, running, climbing and jumping |
|
Feeding |
Reaching for, picking, manipulating, masticating, or placing food in mouth, as well as manipulating the contents of a cheek pouch. |
|
Resting |
Body stationary, usually sitting or lying down. |
|
Social |
Playing, grooming, sexual and aggressive behaviour |
Table 2. List of the types of behavioural categories used in the scan-sampling, with their descriptions(Pombo et al., 2004).
Use of geographic information systems (GIS) and statistical analysis
Global positioning system (GPS) points were collected on site, at areas of high activity and at land marks within the range. The troops’ movements were traced through the day, and marked on hand-drawn maps that would later be used in conjunction with the GPS data, which would allow the daily path-lengths to be calculated using functions of “Arc GIS” and “Idrisi” computer programmes (Bottin et al., 2007). Also, the type of substrates (for example, trees, roof tops, ground and phone lines) that were being used were noted in order to gain a percentage of use for each. The computer programmes SPSS and Microsoft Excel were used for analysing data. Statistical analysis included the non-parametric Mann-Witney U test (as the sample size was relatively small) and Spearman rank correlation. The significance level was set at p≤ 0.05, with levels indicated as p<0.05*, p<0.01** and p<0.001***. Standard deviation was used to show the extent of deviation from the means.
RESULTS
Diet
During the scan-sampling data collection period, the monkeys’ feeding habits were also observed at regular intervals. The types of trees the monkeys fed on were recorded for a three day period for each troop (n = 759), and a percentage was calculated for the ten main trees found to be consumed (figure 3). For Artocarpus heterophyllus (jack fruit) the results were divided between leaves (L) and fruit (F) because monkeys consumed both parts. The column named ‘other’ (figure 3) represents a collection of counts for all the trees consumed not featuring in the top ten. A. heterophyllus fruit was the prime source of food during a six-day period for both troops with a selection of other species (23 for troop A and 18 for troop B) making up the rest. In addition to the food trees mentioned T. v. nestor was also observed, outside of the scan-sampling period, to consume on occasion small flowering plants, creepers, berries, earth from termite mounds and even discarded food from humans.
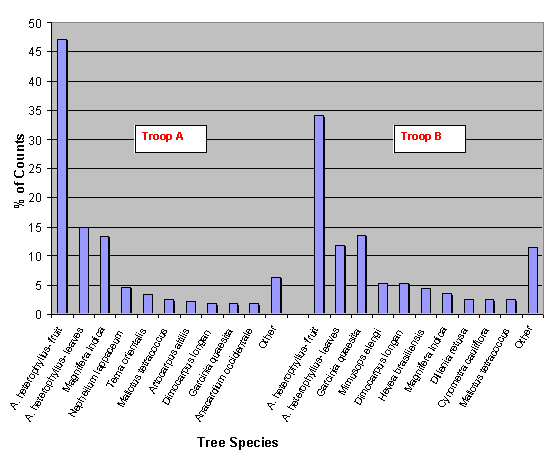
Fig. 3: Bar chart showing the ten main species of trees consumed by troop A and B, and their relative percentages (n=312 for troop A and n=447 for troop B). Jack fruit (Artocarpus heterophyllus) was by far the main food consumed in both troops. In troop A, 14 different species of tree make up the ‘other’ column, therefore, a total of 24 different trees were recorded as being food trees. Nine species of tree make up the ‘other’ column in troop B, giving a total of 19 different trees recorded as being food trees.
Activity budgets
A total of six days’ worth of scan-sampling data (three days for each troop) were used to enable an activity budget to be compiled. Mean values for the different activities participated in were calculated in order to obtain an average daily budget (Table. 3). In addition, the days of observation were divided into four different time bocks, in order to compare differences in activity throughout the day (Figures 4 and 5).
|
|
Moving (%) |
Feeding (%) |
Resting (%) |
Social (%) |
|
Troop A |
7.24±4.73 |
28.48±8.21 |
44.62±10.66 |
19.64±3.38 |
|
Troop B |
10.59±2.99 |
22.74±7.20 |
55.5±10.22 |
11.8±2.69 |
Table 3: Mean daily activity budgets for the two troops (Also showing SD).
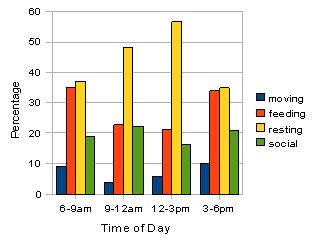
Fig. 4: Activity budget for all individuals in troop A. Percentages for the four categories of activity were calculated over a three day observational period (n=1033). Resting appears to peak in the middle of the day when feeding and moving activities are lower. Social activity appears relatively constant throughout.
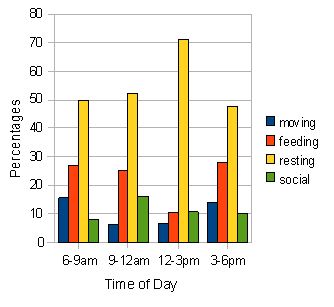
Fig. 5: Activity budget for all individuals in troop B. Percentages for the four types of activity were calculated over the three-day observational period (n=1342). Similar to troop A, resting peaks in the middle of the day when feeding and moving activities fall. Social activity peaks slightly at the 9-12am period.
The two bar-charts (figures 4 and 5) reveal that by far the most frequent activity for the two troops is resting. This peaks at the 12-3pm period for both troops and consists of over 55 % of the activity observed in troop A and over 70 % in group B. Feeding activity appears to be higher in morning and late afternoon for both the troops although this is highlighted more clearly in troop A. Social behaviour is slightly higher in the 9-12am period for both, and moving occurs more in the morning and evening periods for both. The only significant difference found between the various activities in the two troops was in time spent socialising where the results of a Mann-Witney U test (U = 0.00, p < 0.05*).
Daily path-length and substrate use
The mean daily distance travelled for troop A was 654 m (SD±110.95) and troop B was 495 m (SD±208.25) over a seven-day period for each troop (Table 4). The mean value of the two combined was 574.5 (SD±112.43). Troop B’s daily path-length is 32% shorter than troop A. A Mann-Whitney U test revealed a significant difference in distance travelled over roof-tops in the two troops (U= 6.0, p< 0.05*). A Spearman Rank test showed a positive correlation between the distance travelled over roof tops and amount of social activity occurring. (rs= 0.796, p < 0.01**).
|
Troop |
Trees (m) |
Wall (m) |
Roof (m) |
Ground (m) |
Phone Line (m) |
Mean Daily Distance Travelled (m) |
|
A |
484±112.8 |
7±15.7 |
147± 68.0 |
16±16.3 |
0 |
654±110.95 |
|
B |
401±112.2 |
27±43.7 |
52±55.3 |
10±13.1 |
6±10.2 |
495±208.25 |
Table 4: Means of observed substrate use for troop A and B.
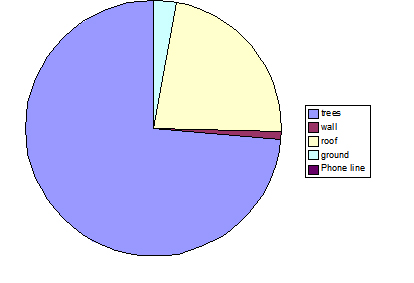
Fig. 6: Percentage of different substrate use, observed over a seven-day period, for troop A. Trees were the main substrate used in travel, utilised 74% of the time; however, roof-tops were also found to be used a substantial amount of the time, making up 23% of the total.
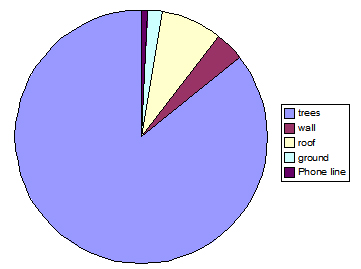
Fig. 7: Percentage of different substrate use, observed over a seven-day period, for troop B. Trees were used 86% of the time, 16% more than troop A, with roof-tops used only 8% of the time. Man-made substrates were used 95% more in troop A than in B.
DISCUSSION
Diet
A previous study conducted by Hladik (1977) on other sub-species of T. vetulus revealed that mature leaves were the main food type consumed. Hladik (1977) found that 60% of the leaves consumed were from only three tree species. However, in a more recent study by Dela (2007) on T. v. nestor in a human-dominated survey area, it appears that this primate sub-species is currently subsisting on fruit from numerous human-cultivated species of tree. In particular, Artocarpus heterophyllus, or jack fruit, appears to make up a significant part of the diet of T. v. nestor. The findings from this current study corroborate strongly with the findings of Dela (2007), since jack fruit was also found to be a main item of food consumed over the study period. Consumption of other fruits from species such as Nephilium lappaceum, Anacardium occidentale and Garcinia quaesita were observed, although they did not constitute enough of the diet enough to class them as a major food source. However, this variety of foods consumed, even in small amounts, does show a level of adaptability to a new environment. Availability and abundance of these particular foods within the range, are probably major influences on amounts consumed (Dela, 2007). This was observed in home range A where the fruit of Nephilium lappaceum, or rambutan, appeared to be a much desired food of the monkeys and they would feast on it whenever possible. However, there was only one rambutan tree present in this range, and it was located in a garden closely guarded by the owner (who regularly chased the monkeys away). Therefore obtaining the fruit was evidently difficult for the monkeys.
The aforementioned study in undisturbed forests by Hladik (1977) show that T. vetulus had a fairly monotonous diet of mature leaves, whereas Dela (2007) demonstrates that under different environmental conditions a more diverse diet is often adopted. This current study recorded a total of 43 different species of tree, a variety of fruits, flowering plants, creepers and even discarded human food, consumed by T. v. nestor, thus suggesting dietary adaptations to a changing, human-modified environment has occurred. According to a study on the Nilgiri langur (a close relative of T. v. nestor) by Sunderraj (1998), primates feeding on a diverse amount of plants indicate a high adaptability to a given home range. Dela (2007) also proposes that T. v. nestor utilises many seasonal plants. However, as this current study was short-term, seasonal differences in diet could not be assessed.
The dietary change witnessed during the study is beneficial in one sense, as it allows T. v. nestor to utilise, and therefore survive in changing surroundings, although the long-term biological consequences on its specialised and highly adapted, leaf-digesting gut will perhaps become a serious concern in the future (Rudran, 2007). In addition, although it appears that the T. v. nestor, is presently coping with its new human-modified environment, these areas are likely to experience further change. Areas close to the study sites were being cleared for new housing throughout the duration of the research and it is only a matter of time before this has a detrimental impact on the primate populations in this area, either through direct habitat loss or effects of isolation.
Activity budget
The results of the activity budget show that the primary activity undertaken by T. v. nestor over the period of observation was resting, followed by feeding, moving and social activities (Table 3). These results are compatible with the findings of Sunderraj (1988), Hladik (1977) and Zhou et al., (2007), who suggest that leaf-eating primates, in particular T. vetulus, minimise energy expenditure in order to cope with the low protein content found in leaves. It is possible, however, that the change in diet of T. vetulus confirmed during the study has affected the activity patterns to some extent. For example, a decline in preferred foods would mean the primate has to search for food outside of its usual range, which is energy costly, or feed more widely on a number of less preferred foods which are easier to obtain (Dela, 2007). Further long-term studies on activity patterns would be necessary to assess this more fully.
The significant difference in socialising time witnessed between the two troops appears to be linked to the distance travelled over man-made substrates, i.e., as distance over roof-tops increased, so too did the social activity. The positive correlation found using a Spearman’s Rank test helped to highlight this link. Troop A were often seen to congregate in large groups on high, flat roof tops for long bouts social grooming. These roof tops were generally found on empty or unfinished houses and therefore did not have inhabitants who would chase them away. They seemed to provide ideal, yet unnatural areas for this type of behaviour and allowed space for grooming between much larger groups of individuals that would perhaps not be possible in the trees. In the range of Troop B, there was only one unoccupied house and the roof was fairly steep which was perhaps unsuitable for bouts of comfortable grooming, thus perhaps helping to explain the lower amount of social activity seen in this troop. This is however a preliminary hypothesis based on small data sets, therefore more studies would be needed to confirm this.
Feeding activity in both troops of T. v. nestor were recorded as being higher in the mornings and evenings whereas resting peaked in middle of the day. These patterns in feeding and resting are consistent with findings in other studies of Trachypithecus species (Sunderraj, 1988; Huang et al., 2002; Zhou et al., 2007). Heightened feeding activities in mornings and evenings are the result of adaptive behaviours. Feeding at these times allows the leaf-eating primates to compensate for the energy lost through the long hours of non-feeding during the night. In addition, the primates can make use of the cooler temperatures at these times, spending the middle of the day, when the temperatures are highest, resting and conserving energy in the shade (Clutton-Brock, 1977; Huang et al., 2002; Sunderraj, 1988).
Daily path-lengths and substrate use
Daily path-lengths of colobines are generally quite short compared withother primates due to the aforementioned adaptive foraging strategy, i.e. minimising energy expenditure to deal with the low-protein intake (Bennett and Davies, 1994; Clutton-Brock and Harvey, 1977). Trachypithecus species, when compared with other colobine species, has one of the shortest daily path-lengths (Kirkpatrick, 2007). Trachypithecus vetulus are known to possess larger forestomachs and sharper molars than other colobines, thus making them more highly adapted to the consumption of mature leaves, which forms the majority of their diet. It is perhaps for this reason their movements during the day are particularly minimal (Kirkpatrick, 2007). The results from the present study reveal that the mean distance travelled for the two troops was 574.5 m (SD±112.43). This figure is consistent with the path-lengths of other Trachypithecus species (Struhsaker and Leyland; 1987, Kirkpartick, 2007). The variation seen between the two troops (i.e. the path-length of troop B was 32% less than A), is perhaps related to amounts of anthropogenic disturbance. On numerous occasions troop A was chased out of certain feeding areas by the owner of the food trees, thus increasing the mean daily path-length. In addition, troop B had one site in particular which they appeared to prefer, and would often spend the whole day in the same region. Here, the presence of an unoccupied house meant they could spend the whole day foraging in the surrounding trees without interruptions. These distances may also be subject to change depending on season/temperature factors, such that distances may be shorter in order to conserve energy when temperatures are high. In addition, food abundance and distribution probably influence the daily-lengths. For example, one sub-site in the range of troop B, where the primates were often present for a number of days without departing, must have contained enough food resources to sustain the troop’s needs for that period of time.
Troops A and B were found to use a variety of different substrates in their daily travels. The majority of time was spent in the trees: however, where tree cover was absent or distances too wide to jump, T. vetulus often utilised other man-made substrates. Troop A was seen to use man-made substrates approximately 95% more than troop B. This increase is probably due to the larger number of houses present in the home range A (almost 50% more). Troop A was often seen congregating on large roofs for bouts of social grooming and this behaviour provides more evidence that T. vetulus could be adapting to a human-modified environment where trees are no longer present.
In natural forest conditions T. vetulus avoids terrestrial locomotion, except for occasional feeding (Roonwal and Mohnot, 1977). The fragmentation of forest evident in Hokandara village has forced T. v. nestor to look for other routes between forest patches. In addition to roofs, phone lines, power-cable masts and walls were used when available, and in the absence of these substrates, the T. v. nestor occasionally travelled over ground. Using these substrates was seen to incur deadly consequences. On 2nd July 2007 at approximately 6 am, villagers reported that a monkey had been electrocuted on a power line. The corpse of the alpha male of troop A was witnessed by the author on arrival at the site. In addition, a week later, another monkey from troop A was killed by a dog when travelling terrestrially. These kinds of deaths have also been reported by Dela (2004), and are likely to become more frequent as the distance between fragments increases due to continuing deforestation.
CONCLUSION
The study presents evidence of the T. v. nestor adapting to a human-modified environment, as shown by variations in substrate use, daily path-length and revised feeding habits. These adaptive abilities have allowed T. v. nestor to survive so far in such fragmented and isolated forest conditions, thus providing a glimmer of hope for this rapidly declining species. However, this study has also noted some areas for concern such as the progressive removal of trees for housing developments, leading to depletion of habitat and food resources, a fact highlighted by the death of two primates whilst crossing the matrix as witnessed in the study.
The activity budget of the T. v. nestor was consistent with other Trachypithecus species; however, the extent to which their activity has been affected by anthropogenic disturbance cannot be established because of the short duration of the study and the relative paucity of related literature. In addition, the study could not consider other factors such as the impact of changing temperatures or lack/abundance of preferred seasonal foods on the behaviour of T. v. nestor due to its short nature. Evidently more longitudinal studies on this species are necessary to corroborate all the information that was assessed here.
ACKNOWLEDGEMENTS
I would like to acknowledge the support of the Undergraduate Research Scholarship Scheme at Oxford Brookes University for funding this project. I would also like to convey my thanks to my supervisor Dr K. A. I. Nekaris for her guidance throughout this project; to C. Eschmann for all the help and companionship whilst in the field; and to G. de Silva Wijeyeratne and the staff of Jetwing Eco Holidays, who were responsible for the logistics of this study. Thanks to K. Conniff and her family for providing accommodation in the field and great tours of the area; to the villagers of Talangama who allowed us access to their land to observe the monkeys and provided me with much vital information needed for the study; to G. Donati, and S. K. Bearder for help their help in various aspects of the analyses.
LIST OF ILLUSTRATIONS AND FIGURES
Fig. 1: Image of Sri Lanka depicting the study site (source: Google Earth).
Fig. 2: Aerial photograph of Hokandara South (source: Google Earth).
Fig. 3: Bar chart showing the ten main species of trees consumed by troop A.
Fig. 4: Activity budget for all individuals in troop A.
Fig. 5: Activity budget for all individuals in troop B.
Fig. 6: Percentage of different substrate use, observed over a seven-day period, for troop A.
Fig. 7: Percentage of different substrate use, observed over a seven-day period, for troop B.
LIST OF TABLES
Table 1: Troop composition for troops A and B.
Table 2. List of the types of behavioural categories used in the scan-sampling, with their descriptions (source: Pombo et al., 2004).
Table 3: Mean daily activity budgets for the two troops.
Table 4: Means of observed substrate use for troop A and B.
NOTES
[1] Richard Moore is now undertkaing an MPhil/PHD at Oxford Brookes. He planned to return to Sri Lanka to continue this research but the political climate in the area has meant that he is instead considering a study of a nocturnal primate called the Tarsier, in Sumatra.
REFERENCES
Bottin, G., M. Huynen and S. Tommaso (2007), ‘Preliminary results on activity budget, feeding ecology and ranging behavior of wild pigtailed macaques (Macaca nemestrina leonina) in Khao Yai National Park, Thailand’, in Vančatová, M and V. Vančata (eds), 2nd Congress of the European Federation for Primatology, Book of Abstracts, Praha: Faculty of Education Publishing House
Clutton-Brock, T. (1977), ‘Some aspects of intraspecific variation in feeding and ranging behavior in primates’ in Clutton-Brock, T. (ed.), Primate ecology: Studies of feeding and ranging behavior in lemurs, monkeys and apes, London: Academic Press, pp. 539–56
Davies, A. and J. Oates (1994), Colobine Monkeys: Their Ecology, Behaviour, and Evolution, Cambridge: Cambridge University Press
Dela, J. (2004), ‘Protecting the endemic purple-faced leaf monkey’, Loris, 23, 14-22
Dela, J. and N. Rowe (2006), ‘Primates in Peril: The World’s 25 Most Endangered Primates, 2004–2006’, Primate Conservation, 20, 1-28
Dela, J. (2007), ‘Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka’, International Journal of Primatology, 28, 607-26
Fleagle, J. (1999), Primate Adaptation and Evolution, 3rd edition,
FRGI (2004), Falling Rain Genomincs Incorporated, Sri Lanka, http://www.fallingrain.com/world/CE/36/Talangama_South.html, accessed 24 October 2007
Groves, C. (2001), Primate Taxonomy, Washington, D.C: Smithsonian Institute Press
Hladik, C. (1977), ‘A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus’, in Clutton-Brock, T. (ed.), Primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys, and Apes, London: Academic Press
Huang, C., F. Wei, M. Li, Y. Li and R. Sun (2002), ‘Sleeping Cave Selection, Activity Pattern and Time Budget of White-Headed Langurs’, International Journal of Primatology, 24 (4), 813-824
IUCN (2001), ‘IUCN List of Threatened Species’, Switzerland: IUCN Gland
Kirkpatrick, R. (2007), ‘The Asian Colobines: Diversity Among Leaf-eating Monkeys’, in Cambell, C., A. Fuentes, M. Mackinnon, M. Panger and S. Bearder (eds), Primates in Perspective, Oxford: Oxford University Press, pp. 186-200
Laurance, W. F., T. E. Lovejoy, H. L. Vasconcelos, E. M. Bruna, R. K. Didham, P. C. Stouffer, C. Gascon, R. O. Bierrgaard, S. Laurance and E. Sampaio (2001), ‘Ecosystem decay of Amazonian forest fragments: a 22-year investigation’, Conservation Biology, 16, 605-18
Mittermeier R. A., N. Myers, C. Mittermeier and R. Gill (1999) Hotspots: Earth's biologically richest and most endangered terrestrial ecoregions, Mexico: Mexico DF CEMEX S.A.
Mittermeier, R., C. Valladares-Padua, A. Rylands, A. Eudey, T. Butynski, J. Ganzhorn, R. Kormos, J. Aguiar and S. Walker (2005), ‘Primates in Peril’, Biological Conservation, 20, 1-28
Nekaris, K. A. I. and G. de Silva Wijeyeratne (2008), The Primates of Sri Lanka, Colombo, Sri Lanka: Jetwing Hotels and Jetwing Eco Holidays under the Jetwing Research Initiative
Parker, L. (2007), ‘Tracking down the elusive western purple-faced leaf monkeys: City slickers seek new home in the country’, Canopy, 5 (1), 19-22
Patterson, J. (2001), Primate behavior: an exercise workbook, Waveland: Prospect Heights
Philip, G. (1991), Philip's Atlas of the World, London, George Philip
Pombo, A., M. Waltert, S. Mansjoer, A. Mardiastuti and M. Mühlenberg (2004), ‘Home Range, Diet and Behaviour of the Tonkean Macaque (Macaca tonkeana) in Lore Lindu National Park, Sulawesi’, in Gerold, G., M. Fremerey and E. Guhardja (eds), Land Use, Nature Conservation and the Stability of Rainforest Margins in Southeast Asia, Berlin: Heidelburg
Roonwal, M., and S. Mohnot (1977), Primates of South Asia: Ecology, Sociobiology, and Behaviour, Cambridge,
Rowe, N. (1996), The Pictorial Guide to the Living Primates,
Rudran, R. (2007), ‘A survey of Sri Lanka's endangered and endemic western purple-faced langur (Trachypithecus vetulus nestor)’, Primate Conservation, 22, 139-44
Strier, K. (2003), Primate Behavioural Ecology, London: Allyn and Bacon
Struhsaker, T. and L. Leyland (1987), ‘Colobines: Infanticide by adult males’, in Smuts, B., D. Cheney, R. Seyfarth, R. Wrangham and T. Struhsaker (eds), Primate Societies, London: The University of Chicago Press
Sunderraj, S. (1998), ‘The ecology of the Endangered Nilgiri Langur (Presbytis johnii) on Mundanthurai plateau, Kalakad Mundathurai Tiger Reserve, Tamil Nadu’, Unpublished Ph.D thesis, Gujurat, India: Saurashtra University
Zhou, Q., F. Wei, C. Huang, M. Li, B. Ren and B. Luo (2007), ‘Seasonal Variation in the Activity Patterns and Time Budgets of Trachypithecus francoisi in the Nonggang Nature Reserve, China’, International Journal of Primatology, 28 (3), 657-71
To cite this paper please use the following details: Moore, R. (2008), ‘Assessment of Diet, Activity Budget and Daily Path-length of Sri Lanka's Endemic Western Purple-faced Leaf Monkey (Trachypithecus vetulus nestor) in a Human Modified Environment', Reinvention: a Journal of Undergraduate Research, Volume 1, Issue 2, http://www2.warwick.ac.uk/go/reinventionjournal/issues/volume1issue2/Moore Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal at warwick dot ac dot uk.
© Reinvention: a Journal of Undergraduate Research (2008). Full copyright remains with the author.