Effect of Air- and Oven-Drying on the Activity of Polyphenol Oxidases and Peroxidases in the Leaves of Gynura procumbens
Jia Jun Fung and Yau Yan Lim, School of Science, Monash University Malaysia
Abstract
Gynura procumbens has been well known for its various medicinal properties such as being an antioxidant. It would be beneficial to be able to dry and preserve the leaves of G. procumbens for commercial utilisation. However, enzymes such as polyphenol oxidases (PPO) and peroxidases (POD) may degrade the antioxidants present. It was found previously that air-drying at ambient temperatures, where PPO and POD activity should be at its optimal temperature, did not significantly reduce the antioxidant activities in G. procumbens. However, oven-drying at 50°C and 100°C reduced the antioxidant properties significantly. Therefore, this study aims to determine the effect of different methods of drying leaves on the enzyme activity of PPO and POD, such as open ambient air-drying, drying in a desiccator at 25% relative humidity, oven-drying at 50°C and 100°C. Results show that all methods of drying that were used fully diminished the enzyme activity of PPO. However, for POD, only oven-drying at 100°C and blanching were able to fully diminish the enzyme activity. Additionally, the desiccator shortened the drying time of open-air-drying by 2–5 days. For commercial utilisation, drying the leaves in a lowered relative humidity would be the best choice.
Keywords
Gynura procumbens, polyphenol oxidase, peroxidase, desiccator, total phenolic content, enzyme activity assay.
Introduction
Drying and its role in food preservation
In the process of drying, water is removed wholly from the product, resulting in the reduction of microbial spoilage and also minimising physical and chemical changes that occur during storage (Zhu and Shen, 2014: 345). This process of dehydration is among the most common as well as the oldest used for preservation in the agricultural and food industry, dating back to c. 8000 BC in south of France (Belessiotis and Delyannis, 2011: 1665). Through the process of food preservation, the food qualities such as shape, colour, taste and texture, and also the nutritional components such as antioxidants, need to be maintained at high levels in order to be beneficial for human consumption (Jin et al., 2014: 172). In addition, many compounds found in food products carry anti-inflammatory, antiviral, antibacterial and anti-cancer properties which are highly valuable to the human health (Martinez-Las Heras et al., 2014: 1–2).
There are various methods used to dry products nowadays, such as freeze-drying, microwave-drying, air-drying, or thermal drying by the sun or using an oven (Atuonwu et al., 2011: 1800). Traditional drying methods usually rely on sun-drying in the open air where the products are subject to external factors such as weather, consumption by other animals and insects, and contamination from contact with animals such as rodents (Pirasteh et al., 2014: 134). Thus, modern drying methods aim to reduce these factors by drying in more controlled environments such as by oven-drying, where the temperature and humidity can be controlled and the time taken for drying is shortened. Furthermore, modern drying techniques – such as solar-assisted heat-pump drying and microwave-hot air oven-drying – have been heading towards combinatorial techniques to help reduce the drying time as well as being more energy efficient (Goh et al., 2011: 4788–89; Schulze et al., 2014: 426–27; Varith et al., 2007: 459–60).
Gynura procumbens
The plant of interest for this study is Gynura procumbens, an annual evergreen plant that is commonly found in tropical regions of south-east Asia, especially Thailand, Indonesia and Malaysia (Rosidah et al., 2008: 616). It is known locally as 'sambung nyawa' which, translated literally, means 'to extend life'.

Figure 1: The Gynura procumbens plant with its green leaves used for this study was grown in Monash University Malaysia. The leaf sizes vary from moderately big to average and are darker green in colour when matured. (Picture taken from author's own collection.)
Traditionally, this plant has been used to treat many illnesses such as eruptive fevers, rash, hypertension, and even cancer (Kaewseejan et al., 2012: 78). As reviewed by Kaewseejan and his team (2012), studies have shown that the plant contains many beneficial properties such as anti-hyperglycemic, anti-inflammatory, anti-Herpes simplex virus, anti-hyperlipidemic, anti-carcinogenic, and anti-ulcerogenic. Furthermore, the plant has been found to contain many antioxidants such as saponins, terpenoids, tannins and the major antioxidants being phenolics and flavonoids (Kaewseejan et al., 2015: 121; Wan et al., 2011: 40–41). A toxicology evaluation by Rosidah et al. (2009) showed that contents in the methanol extract of G. procumbens leaves bear no toxic effects and are safe for consumption, which makes the plant even more valuable.
Although many of these antioxidants are valuable, however, G. procumbens also contains enzymes which can break down the beneficial antioxidants such as the polyphenol oxidases and peroxidases (Akowuah et al., 2009: 81; Hew and Gam, 2010: 2132–36). The presence of these enzymes poses a problem if the leaves of G. procumbens were to be harvested and stored, as the antioxidant content would decrease over time due to the action of the enzymes.
The enzymes
Polyphenol oxidases (PPO) are enzymes known as oxygen oxidoreductases which can be found naturally in plants and seafood, and have many designations such as tyrosinase and catechol oxidase (Sullivan, 2015: 1; Yoruk and Marshall, 2003: 361–62). PPO has been well studied and is found to be the main cause in enzymatic browning in fruits and vegetables by the oxidation of phenolic compounds (Martinez and Whitaker, 1995: 195). As an enzyme and a protein, PPO is also affected by temperature. Yoruk and Marshall's (2003) review on PPO compared PPO activity from several different sources, of which the majority show an optimum operating temperature of around 25–35°C and only moderate heat tolerance with short half-lives between 0.8 to 30 minutes at higher temperatures of around 60–80°C depending on the source of the enzyme.
Peroxidases (POD) are also a member of the class 1 enzyme group of oxidoreductases which are very important in reducing the reactive oxygen species within the cell for survival (Khanna-Chopra and Semwal, 2011: 339–40). PODs have also been shown to be involved in responses to physical wounding of plants (Minibayewa et al., 2015: 123–27). Furthermore, owing to their ability to withstand high temperatures, POD activity is usually used as a reference for the effectiveness of blanching and heat treatments because if PODs cannot survive a heat treatment, other enzyme systems are also very unlikely to survive (Kermasha et al., 1988: 1753).
Main background and importance of the current study
Based on a previous study done by Tan (2015) on drying G. procumbens leaves, there were several results which were deemed to be interesting for further studies which added on to the formation of this current study and its aims. It was found that ambient air-drying resulted in a 13.8% decrease in total phenolic content (TPC) which was lower than oven-drying at 50°C and 100°C, despite the PPO and POD activity at the optimium operating temperature.
Another result of interest is that the oven-drying at 50°C produced an almost similar decrease in TPC as the 100°C oven-dry. Thus, there is a query on whether the decrease in TPC for the 50°C oven-dry is due to thermal degradation of the phenols or enzyme action, or both. For if the decrease in TPC is due to thermal degradation, it would mean that this plant is not suitable to be oven-dried at 50°C and a lower temperature needs to be tested.
The result of the current study would shed light on the conditions appropriate for preservation of the G. procumbens leaves. The knowledge gained would enable the plant to be commercially utilised in terms of mass production for use in the tea industry, dried cooking ingredients industry, or even for the pharmaceutical industry where the leaves can be preserved, stored and exported.
As such, this study will aim to assess the effect of various drying treatments on the oxidase enzymes of this plant, and additionally, provide answers to the results obtained by Tan (2015), particularly on the minor decrease in TPC for ambient air-dried samples. In this study, the drying treatments used are open-air drying and drying in a desiccator at 25% relative humidity at room temperature, oven-drying at 50°C and 100°C. Blanching in boiling water was performed in order to understand the role of enzymes in TPC changes. Following the treatments, assays to determine PPO and POD enzyme activity were carried out. The initial hypothesis was that by oven-drying at 100°C and blanching in boiling water all the enzymes would be denatured, while ambient open-air drying and drying in a desiccator would produce very little change in the activity of the enzymes.
Methods
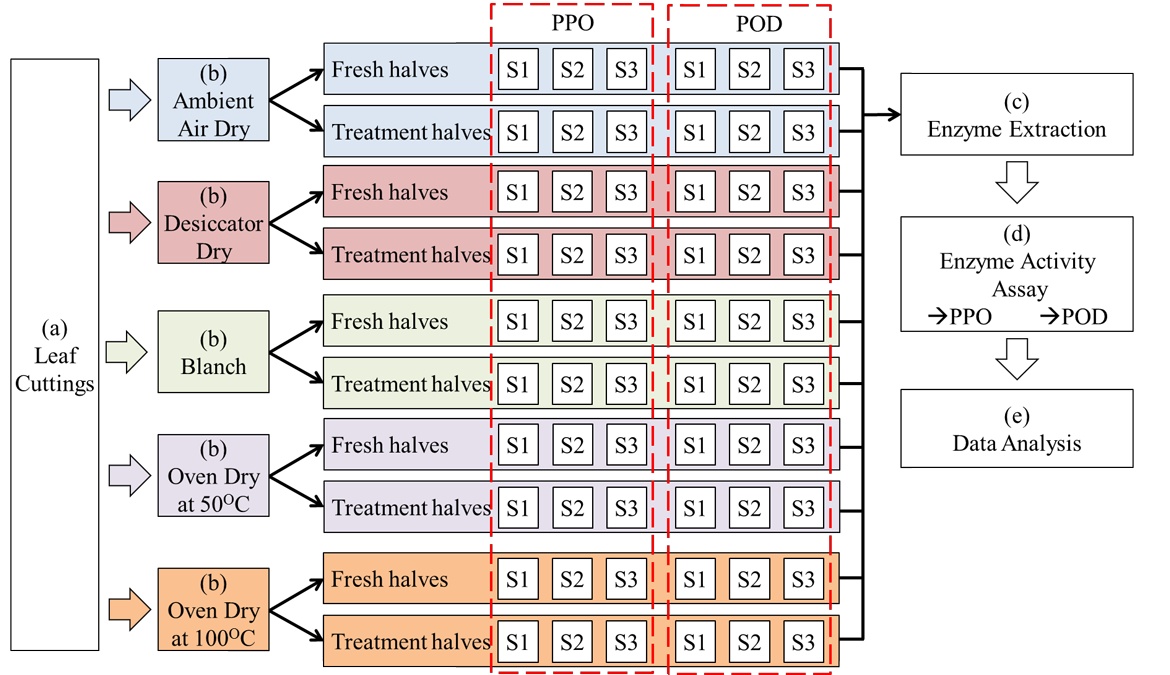
Figure 2: A summary of the entire project process from beginning to end. The sections are labelled as follows: (a) Leaf sample preparation; (b) Treatments; (c) Enzyme extraction; (d) Enzyme activity assay; (e) Data analysis. Abbreviation: S1 = Sample 1; S2 = Sample 2; S3 = Sample 3.
Leaf sample preparation
Mature leaves were cut from the G. procumbens plants and collected fresh on the day of extraction. The half-leaf method was utilised to reduce inter-leaf variation by cutting the leaves in half along the central vein. For the fresh control, halves of different leaves were pooled together to a total weight of about 1.5 g and the matching halves were then pooled together and subjected to a treatment. The cutting and pooling of leaves was done in triplicates for each treatment. The fresh control half-leaves immediately had their enzymes extracted, while the treatment half-leaves had their enzymes extracted after the treatment.
Treatments
For the ambient air-dry treatment, an area in the laboratory away from direct sunlight was cleared and marked to place the samples. A humidity and temperature meter was placed at the area for constant monitoring of the humidity levels and temperature. The leaf samples were placed on respective labelled uncovered petri dishes to dry in laboratory conditions with about 41% relative humidity and air-conditioning 25°C. The weight of each sample was continuously recorded over a period of time until the leaves were dry.
For the desiccator-dry treatment, the same area was used to place the desiccator and it was filled with silica gel at the bottom and closed tightly overnight to allow it to equilibrate to the humidity inside. A humidity-temperature meter was placed inside the desiccator for constant monitoring of the humidity levels and temperatures. The leaf samples were placed on respectively labelled uncovered petri dishes to dry inside the desiccator at around 23% relative humidity and 25°C. The weight of each sample was constantly recorded over a period of time until the samples were fully dry.
For the oven-dry at 50°C treatment, leaf samples were placed on labelled glass petri dishes and put into the oven set at 50°C. The weight of each sample was recorded at 1-hour intervals until the samples were fully dry. The procedure was repeated at 100°C for the oven-drying at 100°C.
The criteria set for determining that the leaves were fully dry were that it would be below 0.13 g (starting from ~1.5 g fresh) and the further decrease in weight was less than 0.01 g. The dried leaves were then subjected to enzyme extraction.
For the blanching treatment, labelled beakers containing 100 mL of distilled water were heated until boiling at approximately 99°C; boiling distilled water was added constantly to maintain the volume at 100 mL. The leaf samples were blanched into the respective beakers of boiling hot water for 60 seconds and immediately removed. The leaves were then subjected to enzyme extraction.
Enzyme extraction
The extraction method was adapted from methods described by Tan et al. (2015). Potassium phosphate buffer (PPB) solution of 0.2 M pH7.0 and 0.05 M pH6.5 was prepared with potassium dihydrogen orthophosphate (KH2PO4) and di-potassium hydrogen phosphate (K2HPO4). Leaf samples were crushed in liquid nitrogen to a fine powder with a mortar and pestle, and transferred into a 15 mL Falcon tube. For the PPO enzyme extraction, crushed samples were suspended in 10 mL of 0.2 M PPB (pH7.0) with 1% PVP-40 (w/v) and 1% Triton X-100 (v/v) at 4°C for one hour under constant shaking condition. For the POD enzyme extraction, the crushed samples were suspended in 10 mL of 0.05M PPB (pH6.5) with 1% PVP-40 (w/v) at 4°C for one hour under constant shaking conditions. The suspensions were then centrifuged at 4,000 x g for 30 minutes at 4°C, and then the supernatant was transferred to another 15 mL Falcon tube and centrifuged at 4,000 x g for additional 15 minutes at 4°C. The final supernatants were stored at –20°C and the enzyme activity was assayed within 7 days.
Enzyme activity assay
The PPO and POD assays were performed as per the methods described by Tan et al. (2015) with minor modifications and adjustments.
The PPO reaction mixture was prepared by dissolving 1 mM catechin in 0.05 M PPB (pH6.5). Using a 24-well microplate, triplicates of 0.2 mL of enzyme extract of each sample were added into the wells of the plate. For the control wells, 0.2 mL of PPB (pH6.5) was used. Then, 1.3 mL of the PPO reaction mixture was added to all wells and immediately placed into the microplate reader to record the absorbance at 435 nm at 30 seconds interval for 30 minutes or until there was no change observed. The absorbance readings were then used to draw the enzyme activity graph, and then the activity was calculated and reported as ΔA435/sec/g of fresh leaves.
The POD reaction mixture was prepared by dissolving 5 mM of tropolone and 3.3 mM of hydrogen peroxide in 0.05 M PPB (pH6.5). The enzyme extracts were diluted by a factor of 8 with POD enzyme extraction solution before use. Using a 24-well microplate, triplicates of 0.05 mL of enzyme extract of each sample were added into the wells of the plate. For the control wells, 0.2 mL of PPB (pH6.5) was used. Then, 1.45 mL of the POD reaction mixture was added to all wells and immediately placed into the microplate reader to record the absorbance at 418 nm at 30 seconds interval for 30 minutes or until there was no change observed. The absorbance readings were then used to draw the enzyme activity graph, and then the activity was calculated and reported as ΔA418/sec/g of fresh leaves.
Results and discussion
Drying process
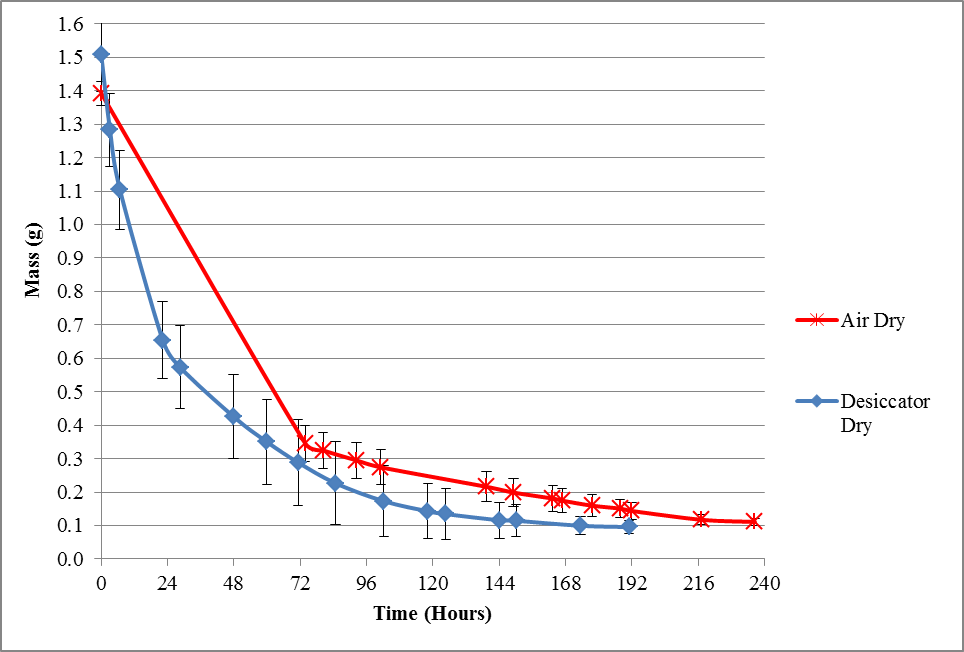
Figure 3: Average decrease in mass of leaves over time for ambient air-dry treatment at relative humidity of 41.5%, and desiccator-dry treatment at 23% relative humidity with an average temperature of 25°C.
Figure 3 shows that the average time taken for the leaves to dry to a constant weight by ambient air-drying took about 10 days (236 hours) while the time taken for the leaves to dry in the desiccator was about 8 days (191 hours). However, the drying time for each individual sample was not consistent. The difference in drying time, within the samples of the same treatment set, is most likely due to the fluctuating relative humidity of the area used for ambient air-drying (Ondier et al., 2010: 547–50). Nevertheless, the result shows that at the same temperature, lower humidity resulted in a shorter drying time.
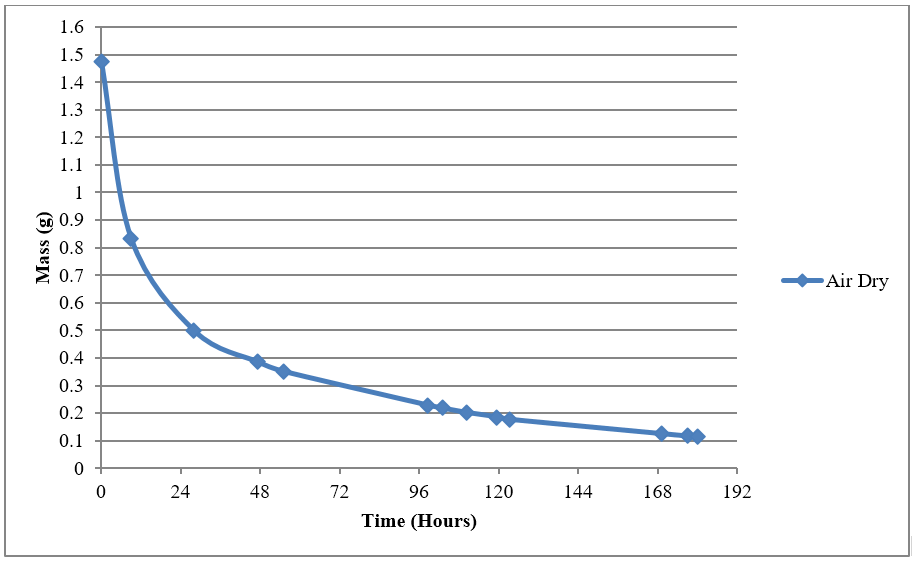
Figure 4: Preliminary work showing the average decrease in mass of leaves over time for ambient air-dry treatment at relative humidity of 40.6% with an average temperature of 25°C.
A preliminary study was done on ambient air-drying and the initial results indicated that at least 3 days were needed for a significant drop in mass when the leaves were ambient air-dried (Figure 4). As such, in Figure 3, the mass readings were not taken during the first 72 hours of ambient air-drying, in order to reduce physical contact with the leaves which might affect the drying process.
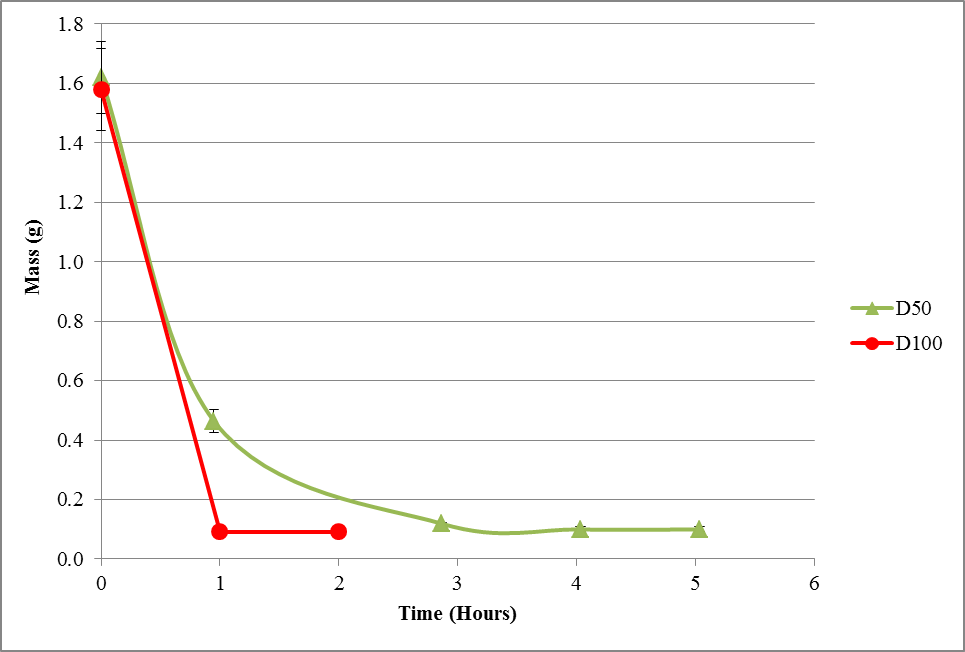
Figure 5: Average decrease in mass of leaves over time for oven-dry treatment at 50°C (D50) and 100°C (D100).
Based on Figure 5, the time taken for the leaves to dry in the 100°C oven was approximately 2 hours while the time taken for the 50°C oven-dry treatment was about 5 hours in total.
The dried leaves appeared to be dull green, non-succulent, and crumpled or scrunched up together.
Ambient air-dry and desiccator-dry treatments
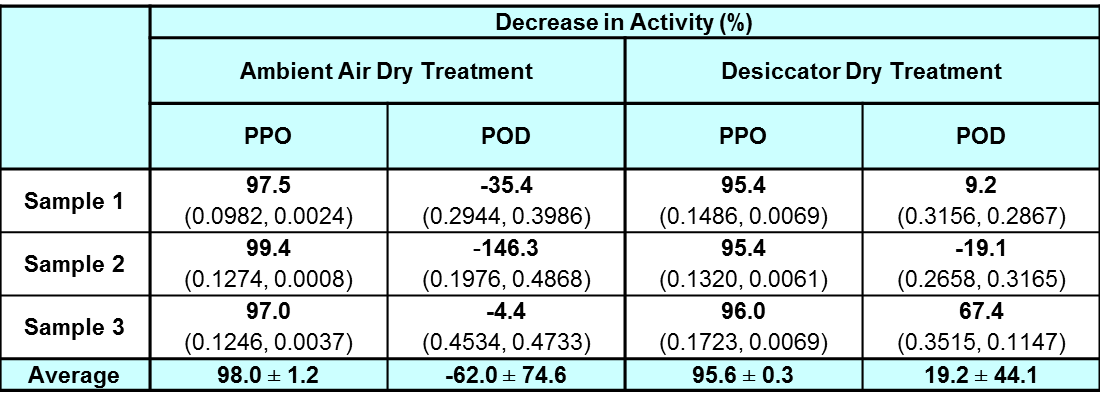
Table 1: Percentage decrease in activity for half-leaf samples subjected to ambient air-dry treatment (236 hours) and desiccator-dry treatment (191 hours) as compared to their fresh halves showing mean ± standard deviation. Negative values show an increase in activity.
Note: The values within the bracket show the enzyme activity values before and after the ambient air-dry treatment and desiccator-dry treatment. The enzyme activity is expressed in ΔA/sec/g of fresh leaves.
From Table 1, after the ambient air-dry treatment, PPO enzyme decreased in activity by 98.0% which is nearly 100%. However, POD activity increased by about 62.0% with one sample showing the largest increase in enzyme activity, of 146%. The same experiment was repeated at another sampling time and the results showed a similar trend of decrease in enzyme activity for PPO and increase in enzyme activity for POD.
The result would indicate that PPO is very heat sensitive, much more so than POD, as even at a temperature of 25°C the activity was nearly 100% lost when air-dried for about 10 days. This may explain why there was a small decrease in TPC when the leaves were air-dried as per the study done by Tan (2015).
On the other hand, POD activity is more stable. Past study suggests that in response to the wounding of the plant, peroxidases are released as a stress response involved in a defence signalling pathway, fixing damaged tissues and also adjusting the plant metabolism (Minibayeva et al., 2015: 123–27). Since the leaves from G. procumbens were physically cut and thus injured, it is very possible that this triggered an increase in production and release of POD within the leaves which led to the increase in POD enzyme activity. Therefore, the decrease in TPC in the study by Tan (2015) is most likely due to the increased POD.
As we have established that the POD enzyme activity increases for the ambient air-dry samples, another point to note would be the small decrease in TPC even though there is high POD enzyme activity. The presence of water in the surrounding environment of the enzyme is necessary for the enzyme to be able to carry out its function (Zaks and Klibanov, 1988: 8017–20). So with the water content in the leaves decreasing over time during air-drying, it is possible that there would not be enough water present for the POD to carry out its function effectively.
In respect of the preservation of the G. procumbens leaves with the aim of maintaining as much TPC within the leaves as possible, the ambient air-dry treatment is a good method as it reduces PPO activity and has a minimal POD activity effect. However, this treatment would depend on the water content of the individual leaves being dried, as high water content would mean that the drying process would be longer and POD would have more time to reduce the TPC present.
From Table 1, after drying in the desiccator at 23% average relative humidity, the PPO enzyme activity decreased by 95.6% on average. However, for POD, the enzyme activity of POD varied greatly but it showed the trend that the decrease was much less than that of PPO. More samplings are needed to find a definite trend.
In general, PPO lost most of its activity on ambient air-drying in an open environment and in a closed environment (in the desiccator). The POD activity could possibly increase or decrease (but less than that of PPO) in a desiccator.
Blanched treatment
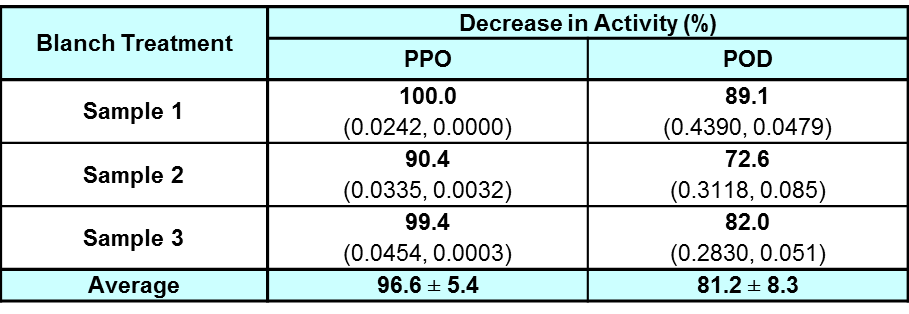
Table 2: Percentage decrease in activity for half-leaf samples subjected to blanched treatment (60 seconds) as compared to their fresh halves showing mean ± standard deviation.
Note: Student's T-test showed that there is significant difference between the decrease in enzyme activity of the data of PPO and POD at α=0.05 (n=3). The values within the bracket show the enzyme activity values before and after the blanch treatment. The enzyme activity is expressed in ΔA/sec/g of fresh leaves.
Based on Table 2, PPO enzyme activity decreased by an average of 96.6% but POD enzyme activity decreased slightly less by 81.2%. Thus, blanching deactivates PPO and POD effectively, but has a stronger effect on PPO.
The lesser decrease in activity of POD could be credited to the fact that the majority of the enzymes in the peroxidase family are naturally heat resistant with examples such as soybean peroxidase, which is thermally stable at temperatures up to 90.5°C, and horseradish peroxidase C is stable up to temperatures of 81.5°C (Friedrich and Pryde, 1984: 227; Henriksen et al., 2001: 109; McEldoon and Dordick, 1996: 555–57). Besides that, there is also the factor of the half-life of the G. procumbens POD which needs to be taken into account. Studies have shown that the denaturation of enzymes does not happen instantaneously but at a certain rate at a certain temperature such as horseradish peroxidase which has a half-life of 121 minute for a 0.02 mg mL−1 concentration of enzyme at 60°C and its iso-enzyme which has 3 times longer half-life (Capone et al., 2014: 857). Therefore, the 1 minute blanching of the leaves might not be long enough to denature all POD present but it also means that the half-life of the G. procumbens POD at 100°C is short, as a short blanching has already reduced activity by over 80%.
Blanching in hot water is a common industrial method used to deactivate enzymes and reduce the microbial presence in food products (Aamir et al., 2013: 11). However, blanching can also lead to deterioration in the quality of the food product such as the reduction of nutrients present in the food product, especially the loss of water-soluble contents through leaching (Xiao et al., 2014: 39).
Oven-dry at 50°C and 100°C treatment
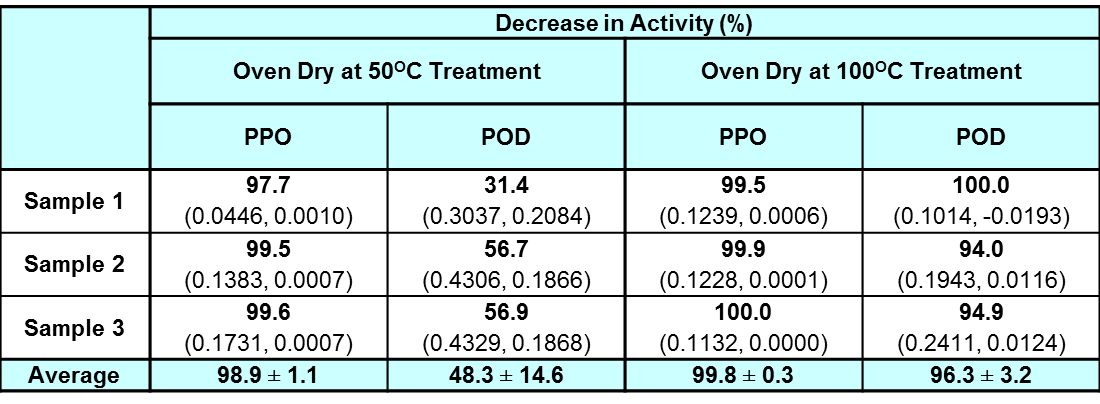
Table 3: Percentage decrease in activity for half-leaf samples subjected to oven-dry at 50°C treatment (5 hours) and oven-dry at 100°C treatment (2 hours) as compared to their fresh halves showing mean ± standard deviation.
Note: Student's T-test showed there is no significant difference between the decrease in enzyme activity of the data of PPO and POD at α=0.05 (n=3) for the oven-dry at 100°C treatment. Student's T-test showed that there is a significant difference between the decrease in enzyme activity of the data of PPO and POD at α=0.05 (n=3) for the oven-dry at 50°C treatment. The values within the bracket show the enzyme activity values before and after the oven-dry at 100°C treatment. The enzyme activity is expressed in ΔA/sec/g of fresh leaves.
Based on Table 3, the enzyme activity of PPO and POD decreased more than 96%. The PPO enzyme activity decreased by more than 99% for all samples, while the POD enzyme activity decreased by more than 99% only for sample 1. However, it is clear that drying at 100°C causes both enzymes to be denatured and unable to carry out its full function effectively.
Since the decrease of both PPO and POD enzyme activity at 100°C is near 100%, the decrease of TPC at 100°C in the study conducted by Tan (2015) can be attributed to thermal degradation and not enzyme degradation. However, the highest temperature at which TPC is stable in the leaves is not known.
Table 3 shows that for the PPO enzyme activity decreased by almost 100% from fresh to dry leaves. However, for the POD enzyme activity, the average decrease was 48% with a large standard deviation. The result again shows that POD is still quite heat stable at 50°C with only a decrease of half of its enzyme activity after 5 hours of incubation.
As per previous point of discussion, POD stems from a family of thermally stable enzymes but the G. procumbens POD is found to be unable to withstand the high temperature of 100°C. Thus, at a lower temperature of 50°C as compared to the 100°C in oven-drying and blanching, it was expected that the enzyme activity would not decrease as much. However, the TPC reduced nearly as much in the 50°C oven-dry as compared to the 100°C oven-dry according to Tan's study (Tan, 2015). In addition to the results from the ambient air-dry treatment, which show that enzymes can still degrade some of the TPC while drying, the decrease in TPC at 50°C oven-dry would most likely be due to enzyme degradation as the enzymes are still functional at 50°C.
A further factor which could influence the decrease in TPC would be the optimum temperature at which POD is active. As enzyme activity increases with temperature until a certain point, and POD is thermally stable, the POD enzyme activity in the leaves could have been much higher at 50°C which allowed it to reduce the TPC much more than when being air-dried at 25°C during the initial period of drying before the water content decreased to a level which inactivates the enzyme.
For the oven-drying treatment with respect to preservation, 100°C oven-dry is not suitable as it reduces a high amount of TPC. However, 50°C oven-dry can be considered for preservation treatment if the leaves were subjected to a pre-treatment such as blanching that could inactivate the enzymes before oven-drying. Even though there will still be some loss of TPC due to the thermal degradation, it is much more economical because the drying time is much shorter than air-dry.
Conclusion
Firstly, regarding the drying time, air-drying the leaves took a comparatively long time (~10 days) and using a desiccator system only shortened it by about 2 days on average; however, it is a promising method as some samples were dry within 5 to 6 days which is half the time taken for open-air drying. Possibly using a better desiccant to lower the relative humidity even further would yield a shorter drying time. In contrast, oven-drying took about 5 hours at 50°C which is much shorter than air-drying. Even though the 100°C oven-drying was much faster (~1 hour), it is not preferred due to the thermal degradation of TPC in the leaves.
Secondly, in terms of enzyme activity, PPO were clearly deactivated by all the treatments carried out but POD were only deactivated by the 100°C oven-dry and blanching in boiling water. Additionally, the 50°C oven-dry results show that there was a decrease in the POD enzyme activity but relatively less compared to the 100°C oven-dry, which was only about a 50% decrease. Besides that, the increase in enzyme activity of POD for the air-dried leaves could possibly be due to increased POD release from the physical damage and heat stress and further testing would be needed to validate it.
Thirdly, in relating back to previous studies on drying and TPC, the lack of decrease in TPC of ambient air-dried leaves is most probably due to the decreasing water content in the drying leaves which therefore does not allow for the enzymes to degrade the phenolic contents. Next, the decrease of TPC in the 50°C oven-dried leaves is most likely due to a mix of both thermal degradation and enzyme degradation. Thus, a pre-treatment to deactivate the enzymes before oven-drying, such as blanching with boiling water, could give less decrease in TPC.
The final verdict with the current set of results would be that drying the leaves in a desiccator with lowered relative humidity, as compared to open-air drying, could be the best choice for industrial use for drying the leaves of G. procumbens. The reason being that drying time was shortened and allowed for decrease in POD enzyme activity as well which would mean less reduction of TPC. As an alternative, it is suggested that a combined treatment of blanching and oven-drying at a lower temperature (<50°C) can also be considered as a good and efficient method of drying G. procumbens leaves.
Future studies
Based on the outcomes of this study, future studies can focus more on determining the best condition (e.g. temperature and relative humidity level) for the desiccator-drying treatment of leaves as this has proved to be the best method thus far for preserving these leaves. As the use of blanching inactivates the majority of the enzymes, it is possible for subsequent studies to combine treatment methods in drying the leaves.
Acknowledgements
Firstly and most importantly, I would like to express my immense gratitude to my supervisor, Associate Professor Lim Yau Yan, for providing me this opportunity to carry out this project under him. His guidance and wisdom imparted is worth more than gold and has certainly impacted me to be more passionate to do research as a career choice. Furthermore, this project has helped me open my mind to vast opportunities to see science in everything around us and allowed me to gain new experiences and practical skills.
Secondly, I would also like to thank Mr Oh Kan Fu (Hikari) and Mr Yew Peng Nian for all the technical knowledge imparted and support given when things did not work out as planned in the laboratory. Their perseverance and interest in science also inspires me.
Notes
Jia Jun Fung is an aspiring biomedical scientist currently pursuing a Master of Science in Biological and Biomolecular Science at University College Dublin.
List of tables
Table 1: Percentage decrease in activity for half-leaf samples subjected to ambient air-dry treatment (236 hours) and desiccator-dry treatment (191 hours) as compared to their fresh halves showing mean ± standard deviation. Negative values show an increase in activity.
Table 2: Percentage decrease in activity for half-leaf samples subjected to blanching treatment (60 seconds) as compared to their fresh halves showing mean ± standard deviation.
Table 3: Percentage decrease in activity for half-leaf samples subjected to oven-dry at 50°C treatment (5 hours) and oven-dry at 100°C treatment (2 hours) as compared to their fresh halves showing mean ± standard deviation.
List of figures
Figure 1: The Gynura procumbens plant with its green leaves used for this study was grown in Monash University Malaysia. The leaves sizes vary from moderately big to average and are darker green in colour when matured. (Picture taken from author's own collection.)
Figure 2: A summary of the entire project's process from beginning to end. The sections are labelled as follows: (a) Leaf sample preparation; (b) Treatments; (c) Enzyme extraction; (d) Enzyme activity assay; (e) Data analysis. Abbreviation: S1 = Sample 1; S2 = Sample 2; S3 = Sample 3.
Figure 3: Average decrease in mass of leaves over time for ambient air-dry treatment at relative humidity of 41.5% and desiccator-dry treatment at 23% relative humidity with an average temperature of 25°C.
Figure 4: Preliminary work showing the average decrease in mass of leaves over time for ambient air-dry treatment at relative humidity of 40.6% with an average temperature of 25°C.
Figure 5: Average decrease in mass of leaves over time for oven-dry treatment at 50°C (OD50) and 100°C (OD100).
References
Aamir, M., M. Ovissipour, S. S. Sablani, and B. Rasco (2013), 'Predicting the quality of pasteurized vegetables using kinetic models: A review', International Journal of Food Science, 2013, 1–29
Akowuah, G. A., A. Mariam, and J. H. Chin (2009), 'The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf', Pharmacognosy Magazine, 5, 81–85
Atuonwu, J. C., X. Jin, G. van Straten, H. C. van D. Antonius, and J. B. van Boxtel (2011), 'Reducing energy consumption in food drying: Opportunities in desiccant adsorption and other dehumidification strategies', Procedia Food Science, 1, 1799–805
Belessiotis, V. and E. Delyannis (2011), 'Solar Drying', Solar Energy, 85, 1665–91
Capone, S., R. Pletzenauer, D. Maresch, K. Metzger, F. Altmann, C. Herwig, and O. Spadiut (2014), 'Glyco-variant library of the versatile enzyme horseradish peroxidase', Glycobiology, 24, 852–63
Friedrich, J. P. and E. H. Pryde (1984), 'Supercritical CO2 extraction of lipid-bearing materials and characterization of the products', Journal of the American Oil Chemists' Society, 61, 223–28
Goh, L. J., M. Y. Othman, S. Mat, H. Ruslan, and K. Sopian (2011), 'Review of heat pump systems for drying application', Renewable and Sustainable Energy Reviews, 15, 4788–96
Henriksen, A., O. Mirza, C. Indiani, K. Teilum, G. Smulevich, K. G. Welinder, and M. Gajhede (2001), 'Structure of soybean seed coat peroxidase: A plant peroxidase with unusual stability and haem-apoprotein interactions', Protein Science, 10, 108–15
Hew, C. S. and L. H. Gam (2010), 'The identification of high abundant proteins in the leaves of Gynura procumbens', Biotechnology and Biotechnological Equipment, 24, 2132–36
Jin, X., R. G. M. van der Sman, G. van Straten, R. M. Boom, and A. J. B. van Boxtel (2014), 'Energy efficient drying strategies to retain nutritional components in broccoli (Brassica oleracea var. italica)', Journal of Food Engineering, 123, 172–78
Kaewseejan, N., D. Puangpronpitag, and M. Nakornriab (2012), 'Evaluation of phytochemical composition and antibacterial property of Gynura procumbens extract', Asian Journal of Plant Sciences, 11, 77–82
Kaewseejan, N., V. Sutthikhum, and S. Siriamornpun (2015), 'Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity', Journal of Functional Foods, 12, 120–28
Kermasha, S., I. Alli, and M. Metche (1988), 'Changes in peroxidase activity during the development and processing of Phaselous vulgaris cv, haricot seed', Journal of Food Science, 53, 1753–55
Khanna-Chopra, R., and V. K. Semwal (2011), 'Superoxide dismutase and ascorbate peroxidase are constitutively more thermotolerant than other antioxidant enzymes in Chenopodium album', Physiology and Molecular Biology of Plants, 17, 339–46
Martinez-Las Heras, R., A. Heredia, M. L. Castello, and A. Andres (2014), 'Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves', Food Bioscience, 6, 1–8
Martinez, M. V., and J. R. Whitaker (1995), 'The biochemistry and control of enzymatic browning', Trends in Food Science and Technology, 6, 195–200
McEldoon, J. P., and J. S. Dordick (1996), 'Unusual thermal stability of soybean peroxidase', Biotechnology Progress, 12, 555–58
Minibayeva, F., R. P. Beckett, and I. Kranner (2015), 'Roles of apoplastic peroxidases in plant response to wounding', Phytochemistry, 112, 122–29
Ondier, G. O., T. J. Siebenmorgen, and A. Mauromoustakos (2010), 'Low-temperature, low-relative humidity drying of rough rice', Journal of Food Engineering, 100, 545–50
Pirasteh, G., R. Saidur, S. M. A. Rahman, and N. A. Rahim (2014), 'A review on development of solar drying applications', Renewable and Sustainable Energy Reviews, 31, 133–48
Rosidah, M. F. Yam, A. Sadikun, and M. Z. Asmawi (2008), 'Antioxidant potential of Gynura procumbens', Pharmaceutical Biology, 46, 616–25
Rosidah, M. F. Yam, A. Sadikun, M. Ahmad, G. A. Akowuah, and M. Z. Asmawi (2009), 'Toxicology evaluation of standardized methanol extract of Gynura procumbens', Journal of Ethnopharmacology, 123, 244–49
Schulze, B., E. M. Hubbermann, and K. Schwarz (2014), 'Stability of quercetin derivatives in vacuum impregnated apple slices after drying (microwave vacuum drying, air-drying, freeze-drying) and storage', LWT – Food Science and Technology, 57, 426–33
Sullivan, M. L. (2015), 'Beyong brown: polyphenol oxidases as enzymes of plant specialized metabolism', Frontiers in Plant Science, 5, 1–7
Tan, H. Y. (2015), 'The effects of oven drying and ambient air drying on the antioxidant and antimicrobial properties of Gynura procumbens leaves', unpublished third year research project thesis, Monash University Malaysia (Under supervision of Dr. Lim, Y. Y.)
Tan, J. J. Y., Y. Y. Lim, L. F. Siow, and J. B. L. Tan (2015), 'Effects of drying on polyphenol oxidase and antioxidant activity of morus alba leaves', Journal of Food Processing and Preservation, 39, 2811–19
Varith, J., P. Dijkanarukkul, A. Achariyaviriya, and S. Achariyaviriya, (2007), 'Combined microwave-hot air-drying of peeled longan', Journal of Food Engineering, 81, 459–68
Wan, C., Y. Yu, S. Zhou, W. Liu, S. Tian, and S. Cao (2011), 'Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures', Pharmacognosy Magazine, 7, 40–45
Xiao, H. W., J. W. Bai, D. W. Sun, and Z. J. Gao (2014), 'The application of superheated steam impingement blanching (SSIB) in agricultural products processing – A review', Journal of Food Engineering, 132, 39–47
Yoruk, R. and M. R. Marshall (2003), 'Physicochemical properties and function of plant polyphenol oxidases: A review', Journal of Food Biochemistry, 27, 361–422
Zaks, A. and A. M. Klibanov (1988), 'The effect of water on enzyme action in organic media', The Journal of Biological Chemistry, 263, 8017–21
Zhu, A. and X. Shen (2014), 'The model and mass transfer characteristics of convection drying of peach slices', International Journal of Heat and Mass Transfer, 72, 345–51
To cite this paper please use the following details: Fung, J. J and Y. Y. Lim (2016), 'Effect of Air- and Oven-Drying on the Activity of Polyphenol Oxidases and Peroxidases in the Leaves of Gynura procumbens', Reinvention: an International Journal of Undergraduate Research, Volume 9, Issue 2,http://www.warwick.ac.uk/reinventionjournal/issues/volume9issue2/fung/ Date accessed [insert date]. If you cite this article or use it in any teaching or other related activities please let us know by e-mailing us at Reinventionjournal@warwick.ac.uk.