Visceral Leishmaniasis
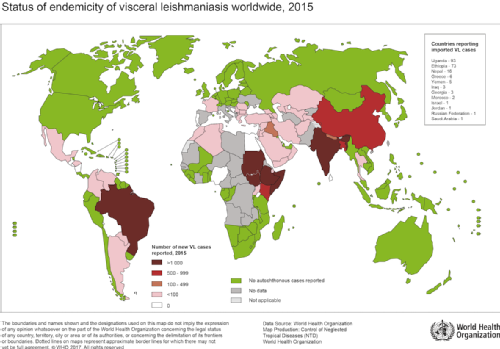
Visceral Leishmaniasis (VL) is the second most deadly parasitic disease in the world following malaria, and is responsible for an estimated 20,000-40,000 deaths worldwide each year. Its distribution is highly localised, with over 90% of the 200,000-400,000 estimated annual cases occurring in just 6 countries: Bangladesh and India in the Indian sub-continent (ISC); Ethiopia, Sudan and South Sudan in Africa; and Brazil.
Map of global VL distribution (WHO, 2015)
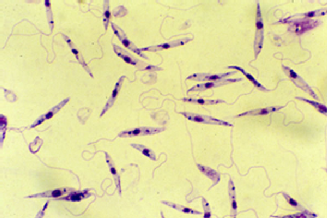
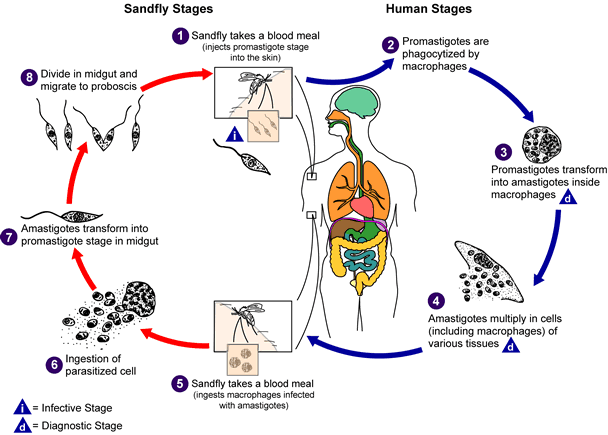
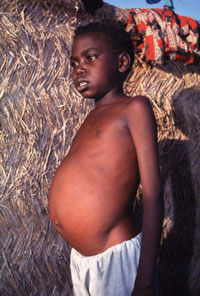
The disease is vector-borne and is caused by chronic infection with protozoan parasites of the Leishmania genus. The parasites are transmitted between humans via the biting of female phlebotomine sandflies. Once inside the body the parasites pass through different stages of their life cycle, reproduce inside macrophages, and migrate to internal organs such as the liver, spleen and bone marrow. Symptoms of the disease include fever, weight loss, anaemia, and substantial swelling of the liver and spleen. If left untreated, the disease is almost always fatal.
Top left: flagellate Leishmania parasite, top right: phlebotomine sandfly, bottom left: swelling of the liver and spleen seen in visceral leishmaniasis, bottom right: life cycle of the Leishmania parasite

Our research focuses on modelling visceral leishmaniasis in the Indian sub-continent, which accounts for the majority of the global burden of the disease. VL in the ISC is caused by Leishmania donovani, and is believed to be anthroponotic (i.e. have no animal reservoir) and spread by a single sandfly species, Phlebotomus argentipes. Consequently, it has been targeted by the WHO for elimination as a public health problem (less than one new case of KA per 10 000 people per year) by 2017. In the rest of the world, VL is zoonotic, limiting the possibility of elimination, and therefore the goal is 100% detection and treatment of all human cases by 2020.
Approximately 80% of VL cases in the ISC occur in the state of Bihar, in north east India, predominantly amongst those who are very poor. Current control strategies are based on sandfly control by indoor residual spraying of insecticide, active case detection and early diagnosis and treatment, but it is not clear whether additional measures are required to achieve elimination.
The majority of individuals infected with the parasite are asymptomatic, i.e. recover from infection without developing clinical symptoms, but are still infectious to sandflies. Determining the role that these individuals play in transmission of VL is of particular concern to the elimination effort and is a focus of our resarch.
Publications
Buckingham-Jeffery, E., Hill, E. M., Datta, S., Dilger, E., & Courtenay, O. (2019). Spatio-temporal modelling of Leishmania infantum infection among domestic dogs: a simulation study and sensitivity analysis applied to rural BrazilLink opens in a new window. Parasites & Vectors, 12(1), 215.
Chapman, L. A., Jewell, C. P., Spencer, S. E., Pellis, L., Datta, S., Chowdhury, R., ... & Hollingsworth, T. D. (2018). The role of case proximity in transmission of visceral leishmaniasis in a highly endemic village in BangladeshLink opens in a new window. PLoS Neglected Tropical Diseases, 12(10), e0006453.
E.A. Le Rutte L.A.C. Chapman L.E. Coffeng, J.A. Ruiz-Postigo, P.L. Olliaro, E.R. Adams, E.C. Hasker, M.C. Boelaert, T.D. Hollingsworth, G.F. Medley, S.J. de Vlas (2018). Policy Recommendations From Transmission Modeling for the Elimination of Visceral Leishmaniasis in the Indian Subcontinent. Clinical Infectious Diseases, 66 (suppl_4):S301–S308.
S. Jervis, L.A.C. Chapman, S. Dwivedi, M. Karthick, A. Das, E.A. Le Rutte, O. Courtenay, G.F. Medley, I. Banerjee, T. Mahapatra, I. Chaudhuri, S. Srikantiah and T.D. Hollingsworth (2017). Variations in visceral leishmaniasis burden, mortality and the pathway to care within Bihar, India. Parasites and Vectors, 10:601.
E.A. Le Rutte, L.A.C. Chapman, L.E. Coffeng, S. Jervis, E.C. Hasker, S. Dwivedi, M. Karthick, A. Das, T. Mahapatra, I. Chaudhuri, M.C. Boelaert, G.F. Medley, S. Srikantiah, T.D. Hollingsworth, S.J. de Vlas (2017). Elimination of visceral leishmaniasis in the Indian subcontinent: a comparison of predictions from three transmission models. Epidemics, 18:67-80.
L.A.C. Chapman, L. Dyson, O. Courtenay, R. Chowdhury, C. Bern, G.F. Medley and T.D. Hollingsworth (2015). Quantification of the natural history of visceral leishmaniasis and consequences for control. Parasites and Vectors, 8:521.
M.M. Cameron, A. Acosta-Serrano, C. Bern, M. Boelaert, M. den Boer, S. Burza, L.A.C. Chapman, A. Chaskopoulou, M. Coleman, O. Courtenay, S. Croft, P. Das, E. Dilger, G. Foster, R. Garlapati, L. Haines, A. Harris, J. Hemingway, T. Déirdre Hollingsworth, S. Jervis, G. Medley, M. Miles, M. Paine, A. Picado, R. Poché, P. Ready, M. Rogers, M. Rowland, S. Sundar, S.J. de Vlas and D. Weetman (2016). Understanding the transmission dynamics of Leishmania donovani to provide robust evidence for interventions to eliminate visceral leishmaniasis in Bihar, India. Parasites and Vectors, 9:25.
K.S. Rock, G. Medley, R. Quinnell, O. Courtenay (2016). Progress in the mathematical modelling of visceral leishmaniasis. Advances in Parasitology, 94:49-131.
G.F. Medley, T.D. Hollingsworth, P.L. Olliaro, E.R. Adams (2015). Health-seeking behaviour, diagnostics and transmission dynamics in the control of visceral leishmaniasis in the Indian subcontinent. Nature Supplement, 528:S102–S108.
K.S. Rock, E.A. le Rutte, S.J. de Vlas, E.R. Adams, G. Medley, and T.D. Hollingsworth (2015). Uniting mathematics and biology for the control of visceral leishmaniasis. Trends in Parasitology, 31 (6):251-259.
Funded by: Bill and Melinda Gates Foundation
People involved:
Collaborators:
Emily Adams (LSTM)
Caryn Bern (UCSF)
Lloyd Chapman
Deirdre Hollingsworth
Graham Medley
Piero Olliaro (WHO TDR)
