Graphene Oxide
One of our primary focuses is in the area of the chemistry of graphene oxide. We, together with researchers led by Neil Wilson in the microscopy group in Physics have been investigating the structure, properties and uses of graphene oxide (GO). Our current work is on functionalising GO.
A highlight is the discovery that GO, as produced, is heavily contaminated with oxidative debris (OD). This debris may easily be removed by washing with a solution of sodium hydroxide, giving a black material (bwGO) that is much more graphene like.
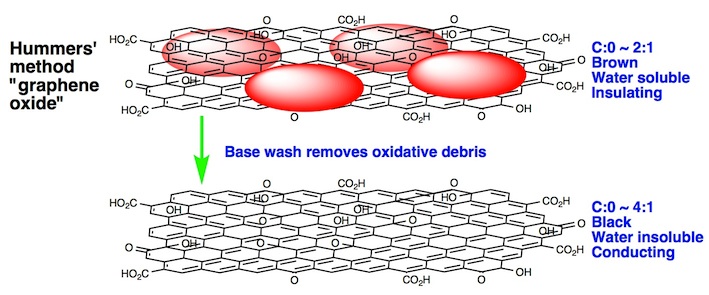
See our "hot paper" in Angewandte Chemie.
This paper has provoked a lot of discussion (325 citations to date), and we have recently published a defence of our model. See our defence.
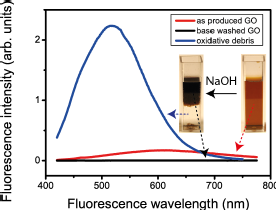 |
We have shown that the separation of oxidation debris from GO has a profound effect on the fluorescence: the bwGO is no longer fluorescent but the debris fluoresces more intensely, blue-shifted relative to the unwashed GO. |
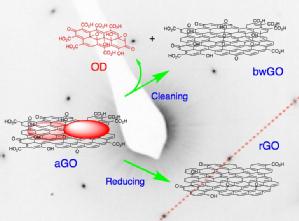 |
We have also shown that the two component model (graphene like sheets with oxidative debris) applies to graphene oxide, irrespective of the route used to synthesise it. |
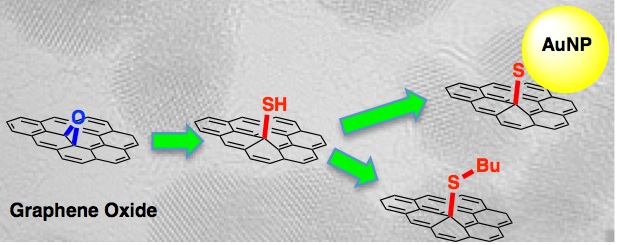 |
More recently we have shown how it is possible to functionalise the GO with surface thiols, and then to attach new chemical groups. TEM alowed us to directly image attached gold nanoparticles. |
|
We have also anchored individual Au9 clusters to the GO surface and directly imaged them, as well as observe their individual motion |
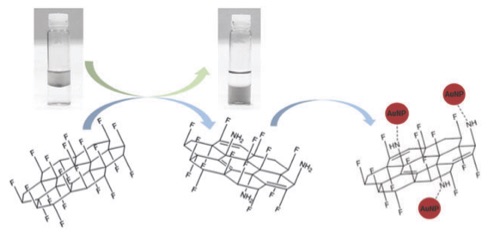 |
Work wih fluorographite shows how it may be functionalised and hence utilised. |
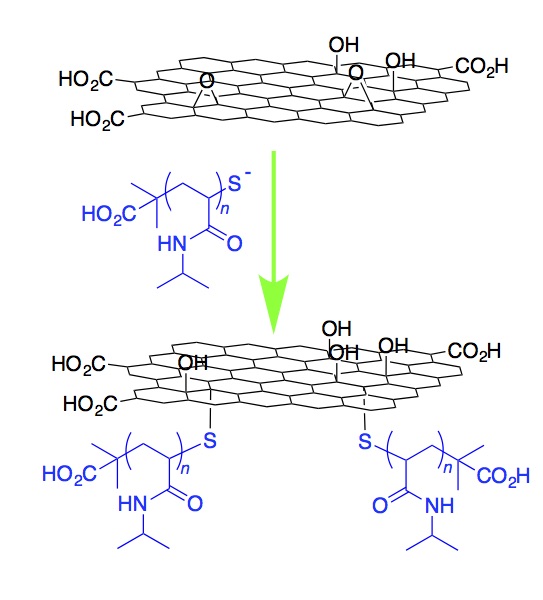 |
The direct grafting of poly(N-isopropylacrylamide) to the basal plane of graphene oxide has been achieved in a single step: cleavage of the terminal thiocarbonylthio group on RAFT grown poly(N-isopropylacrylamide) reveals a reactive thiol that attacks the epoxides present across the surface of graphene oxide. A wide variety of composite materials can be synthesised this way. |
|
Electrodeposition of nanoparticles on graphene provides a convenient approach for making graphene–nanoparticle composites as well as insight into the electrochemical activity of graphene. CVD grown graphene was used directly on its copper growth substrate, and we identify and isolate two nanoparticle growth processes for both silver and palladium deposition: electroless deposition that appears to occur preferentially at defects and edges next to the underlying copper, and conventional electrodeposition that occurs uniformly The resultant nanoparticles are homogeneously dispersed across the graphene surface, suggesting that here both edge-plane and basal-plane graphene sites are electroactive. |
|
|
Two different pyrene-substituted ions were used to render the surface of graphene hydrophilic. Self- limiting monolayers of ammonium and sulfonate substituted pyrenes were used to give,respectively, an overall positive and negative charge to the surface. Both pyrenes gave a stable hydrophilic surface and were used to selectively immobilise negatively or positively charged macro-molecules. This simple and versatile non-covalent approach can be used on graphene on a variety of substrates (e.g. copper, SiO2), suspended graphene, and also for graphite |
Other published work on GO includes the seminal paper on its use as a highly transparent support for electron microscopy, as well as work utilising GO (publications 34, 35, 40 and 42).

