Information for patients
If you have experienced pregnancy loss and would like help or advice please click here for resources.
Recurrent miscarriage (two or more miscarriages in a row) causes considerable distress for women and their partners. The vast-majority of couples receive supportive care only, as few treatments have been shown to prevent miscarriage. The hope is that this trial may help those who currently have no explanation for the reason they have experienced recurrent miscarriage.
Information about the trial
| What is the purpose of the research trial? |
| The aim of the CERM trial is to find out if antibiotics can reduce miscarriage. In some women the lining of the womb (the endometrium) is inflamed (a condition called endometritis). Researchers have found a link between this and miscarriage. A healthy endometrium is important for the embryo to be able to attach to the womb. Treating endometritis with antibiotics may reduce the inflammation and the likelihood of a miscarriage. This research trial will test this theory by comparing a 14-day course of an antibiotic (doxycycline) against a placebo to find out if taking antibiotics reduces miscarriages. |
| What does the trial involve? |
Whether you will receive doxycycline or the placebo is decided by chance.
|
Is the trial recruiting? |
| The CERM trial is not open to new patients and has completed final recruitment. |
| Below is a diagram showing an overview of the CERM trial: |
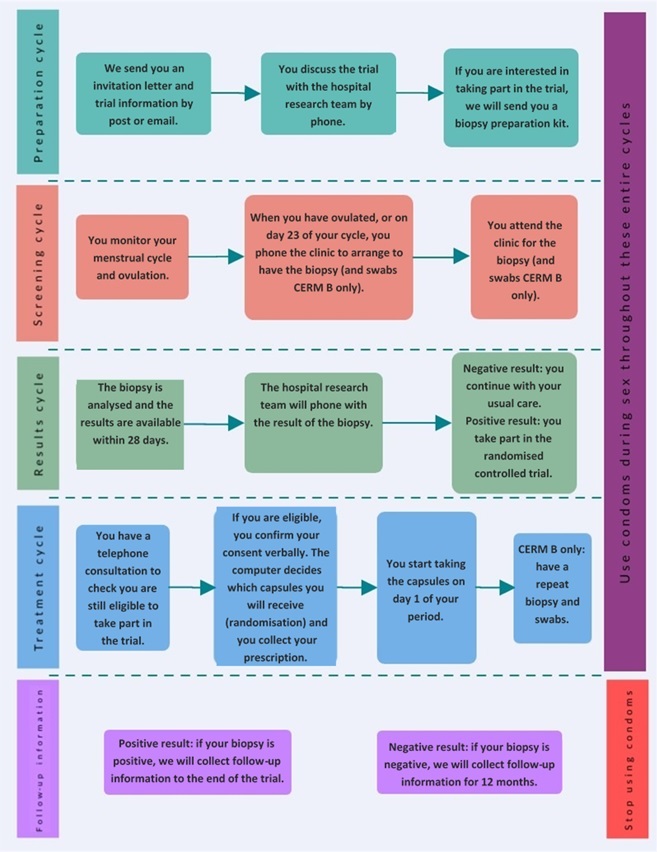
Please note: 'CERM B' activities only relate to participants at University Hospital Coventry & Warwickshire (who have consented to CERM B) |

Enquiries:
Email:
Address:
Warwick Clinical Trials Unit
Warwick Medical School
University of Warwick
Gibbet Hill Road
Coventry
CV4 7AL
