Trial overview
Study Summary:
In the UK, 35,000 patients have a cardiac arrest in hospital each year. Unfortunately, less than one in five of these patients survives to leave hospital. Chest compressions are an essential treatment for cardiac arrest patients, but can be difficult for a person to deliver to a high standard (manual chest compressions). A mechanical chest compression device can be used to deliver chest compressions (mechanical chest compressions) instead of a person. Small studies suggest using these devices may improve patient survival when used as part of treatment for cardiac arrest patients in hospital.
We wish to test this in a large trial but this will be costly and logistically challenging, so we plan to first undertake a small study. This is called a feasibility study and will help to demonstrate how a larger trial could be carried out effectively and successfully.
This small study will take place in three hospitals over two years. 330 patients that have a cardiac arrest will participate. After initial treatment has been started (including manual chest compressions), patients will either continue to receive manual chest compressions or they will receive mechanical chest compressions. The treatment will be decided randomly. All patients will receive all other necessary treatments.
For this small study, we will look at a number of things including how many patients take part, how well devices are deployed, and how many participants agree to complete the follow-up questionnaires. We will also collect information about the patient's hospital stay and see how they recover after leaving hospital.
Alongside this, we will interview NHS clinicians to identify factors that help or hinder study recruitment (qualitative component of the study).
This study will provide important information about the practicality of undertaking a large trial, and may provide some early information about whether these devices are beneficial to use for patients that have a cardiac arrest in hospital.
This study is funded by the National Institute of Health Research.
The study protocol can be downloaded here: COMPRESS-RCT Protocol ![]()
What is the main aim of the study?
The aim of this study is to find out whether it is feasible to carry out a larger study to test whether there is any significant difference between applying CPR (chest compressions) with a mechanical device compared to standard manual CPR for 30 –day survival and future quality of life for the patient.
Who can participate?
Patients will be eligible to take part in the study if they are over 18, have a cardiac arrest in one of the study hospitals and resuscitation is attempted by a team trained in the use of a mechanical chest compression device. Patients must also be in a non-shockable rhythm.
Some patients will not be able to participate, such as those that are pregnant or where use of a device could be harmful to the person.
Where is the study taking place?
We plan for the study to take place at the following NHS hospitals in the West Midlands:
-
Birmingham Heartlands Hospital (Heart of England NHS Foundation Trust)
-
University Hospital, Coventry (University Hospitals Coventry and Warwickshire NHS Trust)
-
Sandwell General Hospital (Sandwell and West Birmingham Hospitals NHS Trust)
- Warwick Hospital (South Warwickshire NHS Foundation Trust)
What is a mechanical chest compression device?
A mechanical chest compression device is an electrically powered machine which has been developed to deliver chest compressions on patients who are in cardiac arrest, instead of this being done by a person.
In this study we are using the LUCAS device. This device consists of a piston that presses down on the chest of the patient to deliver chest compressions. The LUCAS device has been approved to meet European health and safety requirements.
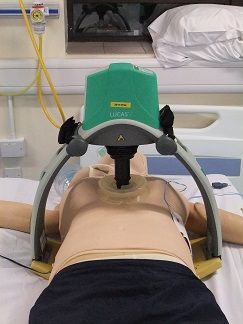
What does the study involve?
Following a cardiac arrest in hospital, participants will be randomised to receive either mechanical chest compressions or to continue to receive manual chest compressions.
All trial participants will receive manual chest compressions initially. In patients randomised to the mechanical chest compression device, the device will be used as soon as possible after randomisation.
All study participants will be treated by the hospital cardiac arrest team, which comprises clinicians skilled in the delivery of cardiac arrest care in accordance with Resuscitation Council (UK) cardiac arrest guidelines. All standard resuscitation interventions will be delivered including chest compressions, advanced airway management, defibrillation, and drug therapy.
Participants who survive their cardiac arrest will be contacted by local research staff and asked if they would be willing to allow researchers to collect information from their medical records for up to six-months after their cardiac arrest.
Participants will also be asked if they would be willing to complete a study questionnaire before they are discharged from hospital and at six-months after their cardiac arrest.
We will also undertake interviews with NHS staff involved in the study. These interviews will explore the participant’s experience of the study and how this compares with their experience of other clinical research.
When is the study taking place?
The anticipated recruitment start date for the study is late January 2017. The recruitment period will last approximately two years.
We plan to report the study results by the 30th September 2019.
If you would like information about the study results when they are published, please contact the COMPRESS-RCT Trial Coordinator at Warwick Clinical Trials Unit via email or telephone.

Enquiries:
Please direct all general enquiries to:
Tel: 02476 150478
Email: compresstrial at warwick dot ac dot uk
