Vector Transmission
A vector-borne disease (specifically a biological transfer rather than mechanical) is one in which transmission of infection in a population (the host population) occurs only via a second population (vectors). Vectors are usually haematophagous (blood-feeding) arthropods such as mosquitoes, sandflies or ticks. There are many such diseases, the dynamics of which are of great interest from the point of view of controlling infection. Malaria, leishmaniasis, dengue fever and west Nile virus are but to name a few which currently have a huge social impact on human populations.
Each year in the region of 225 million people are infected with the malaria parasite and in 2009 around 781,000 of these resulted in disease-induced mortality. The story is similar for leishmaniasis where there are around 12 million people globally who are thought to be infected at any given time and about 80,000 people die annually from the most serious form, visceral leishmaniasis. Such diseases are rife in the developing world; a combination of optimal habitat for vectors in tropical regions and a lack of medical aid lead to large-scale endemics there.
Human diseases are not the only concern; there are many others that infect other classes of hosts which are also of interest, particularly from the point of view of preserving endangered species.
The typical course of infection for vector-borne transmission, ignoring disease, host or vector specics, starts with an infected vector (a blood feeding or sucking adult arthropod) taking a blood-meal from a susceptible member of the host population. Once bitten, the host has a chance to become infected with the disease. At this stage, if infected, the host is considered exposed but suers no adverse eects from the infection and cannot transmit the disease. The time after becoming infected but before becoming infectious is known as the latency period. When the parasite has reached a certain stage in its reproductive cycle, the host becomes infectious after which, if it is bitten, it can transmit the disease to a susceptible vector through its parasite-infected blood and completing the transmission cycle (shown in the figure below).
Disease transmission is usually characterised by:
- abundance and spatial spread of both hosts and vectors
- demography
- a feeding rate or vector desire to bite
- success or failure of transmission
Typically mathematical models of vector-borne diseases are based upon a two-population SI(R) model exhibiting “criss-cross” infection terms, where and
are the force of infection of terms for hosts and vectors respectively:
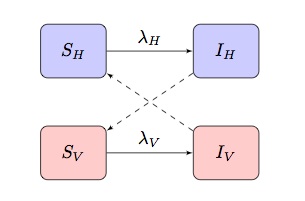
Dependent upon the disease and population being modelled, this may be adapted to incorporate other more specific features.
Modelling the Dynamics of Vector-Borne Diseases
There are many different types of mathematical model for vector-borne disease transmission; ideally these are sought to be kept mathematically tractable and as simple as possible whilst retaining the general dynamics of the biological system. Unsurprisingly models from the literature range greatly; from deterministic to stochastic, endemic (including birth and death rates) or epidemic (an outbreak of limited duration), incorporating spatial spread, spatial heterogeneity, latency periods, age structure, acquisition and loss of immunity, multiple strains and many others. Even when only considering just one specific disease the variations in the disease within different populations may lead to changes in not just the parameterisation, but also of how one may wish to take the modelling approach.
Some of Kat’s work focuses on the simplifications that are often made to reduce the complexity of models. This work considers the effects of using "host-only" transmission models such as the quasi-equilibrium assumption upon disease dynamics and also the effect of omitting the latency period upon epidemic predictions.
Leishmaniasis
To be completed by Erin
Human African Trypanosomiasis (HAT)
Human African trypanosomiasis (HAT), more colloquially known as sleeping sickness, is a deadly disease which is endemic across much of sub-Saharan Africa. 70 million people live in at risk areas covering over one and a half million square kilometres. Whilst the prevalence of HAT is not as high as that of other vector-borne diseases such as malaria or dengue (there were just over 7000 reported cases of HAT but 207 million estimated malaria cases in 2012), the lack of chemical prophylaxis, the extremely unpleasant (and often deadly) treatment and the shortage of substantial scientic research has now placed HAT on the WHO's list of neglected tropical diseases.
The vector for HAT is the tsetse; its need to blood feed to prevent starvation and a unique vector-parasite interaction (causing a "teneral susceptibility phenomenon") mean that HAT modelling is distinctly different to other vector-borne diseases such as malaria.
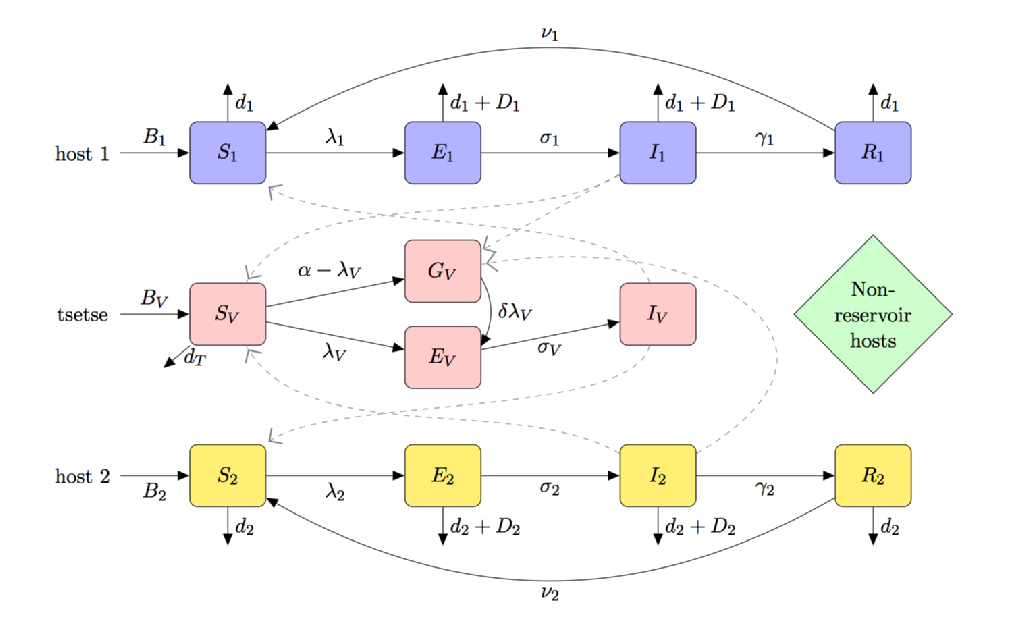
The figure below is a compartmental caricature of a model for HAT which includes:
- (partial) teneral fly susceptibility
- multi-host species (including reservoir and non-reservoir hosts)
- tsetse feeding preference
- starvation of unfed tsetse
Bluetongue Disease (BTV)
Bluetongue is a viral disease (BTV) of ruminants transmitted by various Culicoides genus biting midge species. Symptoms for livestock diseased with BTV included discomfort, high fever and cyanosis of the tongue, which gives the disease its name. Amongst sheep BTV disease has a high associated mortality. Until the late 1990s the European range of BTV was entirely associated with the range of the midge vector C. Imicola. At the end of the '90s and into the early 2000s there has been a previously unprecedented expansion of BTV into areas of Europe which are part of the range of other midge species such as those of the C. Obsoletus complex and closely related midge species. In particular an outbreak originating in the Netherlands in 2006 is the first recorded example of a BTV incursion to the 50 degrees north latitude. In subsequent summers BTV cases were discovered across Northern Europe including in Belgium, Germany, UK and Denmark demonstrating the capability of Bluetongue to over-winter in the Northern European environment. A puzzling feature of the Northern European serology of Bluetongue is that disease was caused by a distinct strain (BTV-8) from those responsible for incursions into Southern Europe i.e. Spain (BTV-4) and Italy (BTV-2).
Predicting the impact of BTV incursion and best design of livestock management and disease control requires cutting edge modelling methodology. A great deal is understood about the epidemiology of BTV such as a solid understanding of the expected period of viraemia for cattle and sheep hosts and the effect of climatic temperature upon the incubation period of Bluetongue within its midge vector. However, estimating the spatio-temporal risk posed by vector dispersal away from infected host livestock remains a significant challenge. The inference problem here is multi-factorial requiring not only parameter inference but also statistically principled model selection.
Malaria
Malaria follows the generic transmission cycle as previous described; the vector being the female mosquito who takes her blood meal as part of the reproductive process; the males of the species are nectavorious and as such play no part in the transmission cycle. The frequency of feeding is determined by this biological need, and so the average rate of feeding is usually around once every four days and it is usually assumed that satiation for one batch of eggs is achieved within this one meal. There are many different types of malaria, some affecting humans (this may be caused by one of four different types of Plasmodium) and others affecting animals. The genus Anopheles is responsible for transmission to humans, of which approximately only 30 out of 400 species predominantly cause the spread of infection.
A key feature affecting malaria is temperature; even if the mosquito resides within the given area, external temperatures of below about 16C (exact temperate is dependent on the species of parasite), it is not warm enough to allow development. In general, as the temperature increasing from 16C the shorter the incubation period with a sudden halt above about 32C as the parasite is not able to survive past this temperatures.
Avian Malaria and the Hawaiian Honeycreeper
Some systems, however, may be far more complex than the general example given previous. One such example is the Hawaiian Honeycreeper (Drepanididae); it suffers not just the adverse effects from Avian Malaria (Plasmodium relictum) which is transmitted via the mosquito (Culex quinquefasciatus) but also those of climate change, and predation. The interplay between these dominating factors is great, with temperatures and abundance of predators affecting the course of the disease and the birds’ ability (or inability) to resist extinction. Furthermore some species of the honeycreeper exhibit the ability to confer resistance to malaria, suffering no deleterious effects from the disease whilst remaining infectious (these are more commonly referred to as carriers and inadvertently cause a reservoir of infection by surviving the normal “disease-free” life expectancy but still spreading infection.
This work has been published as:
K. S. Rock et al. "Modelling the Future of the Hawaiian Honeycreeper: An Ecological and Epidemiological Problem". Ecological Modelling (June 2012)
Funded by: EPSRC, EU / DEFRA, BMGF
People involved:
Matt Keeling
Orin Courtenay
Deirdre Hollingsworth
Kat Rock
Sam Brand
Lloyd Chapman
Mike Irvine
External Collaborators:
Steve Torr (LSTM)
Emily Adams (LSTM)
Jon Edmunds (LSHTM)
Raph Metras (LSHTM)
Uno Wennergren (Linkoping)
Vittoria Colliza (U Pierre et Marie Curie)
