Dynamical signalling NF-KappaB
I am particularly interested in signalling systems that display dynamic behaviour. There are many such systems of great importance and an open question is whether the dynamics is functional and enables the systems to do things that would be difficult or impossible in equilibrium systems.
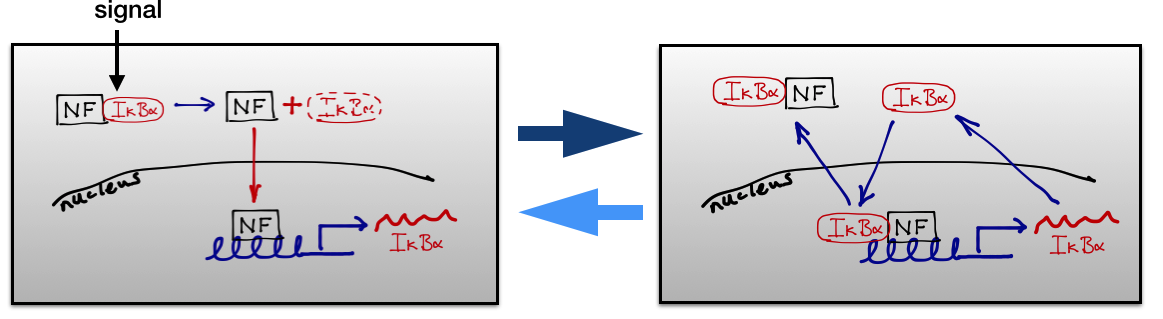
An exemplar is the NF-kappaB system which is one of the most important stress response pathways in the mammalian cell. My work in this area is almost all in collaboration with Mike White (Manchester). Most of our work is concerned with understanding the function of the oscillations in the NF-κB system, whereby the transcription factor NF-κB locates in and out of the nucleus in a periodic fashion when the system is activated. These oscillations were discovered in Mike's lab, initially in cell cultures but now in primary cells as well.
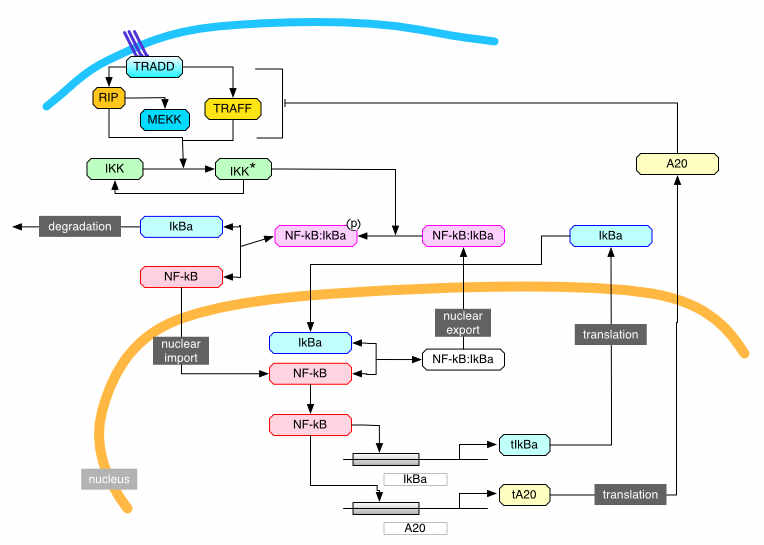
My basic hypothesis in this area is that NF-κB acts as an information hub with the oscillations allowing it to carry much more information than would be possible otherwise. I am particularly interested in developing a conceptual framework for understanding the transmission of information in signalling systems. In a recent lecture at the Royal Society on the Great Ideas of Biology, Sir Paul Nurse speculated that if we want to understand how biological organisms work then we need to explain the higher-order phenomenon of living organisms by relating the chemical and physical processes to the processing of information and the way that information is used to determine outputs. He quoted Francis Crick in his argument that it is better in biology to follow the flow of information than those of matter or energy. Moreover, he specifically highlighted the importance of dynamics because dynamical networks can transfer much more information. As he said, thinking of biology as an organised system processing information is an embryonic endeavour whose pursuit will crucially need the help of physicists and mathematicians. The problem is that we are missing an adequate conceptual framework for discussing genomic information once it has been passed into the stochastic dynamic interactions that make up cellular processes.
I am preparing a paper with George Minas and Dan Woodcock that provides the mathematical background to this hypothesis. For this theory we needed a new stochastic approximation of biological oscillators that addresses the need for both analytical tools and also algorithms for accurate and fast simulation and estimation of stochastic oscillator systems. A recent paper presents our method, called phase-corrected LNA (pcLNA), that overcomes the main limitations of the standard Linear Noise Approximation (LNA) to remain uniformly accurate for long times, still maintaining the speed and analytically tractability of the LNA. As part of this, we develop analytical expressions for key probability distributions and associated quantities, such as the Fisher Information Matrix and Kullback-Leibler divergence and we introduce a new approach to system-global sensitivity analysis. We also present algorithms for statistical inference and for long-term simulation of oscillating systems that are shown to be as accurate but much faster than leaping algorithms and algorithms for integration of diffusion equations. Stochastic versions of published models of the circadian clock and NF-κB system are used to illustrate our results.

