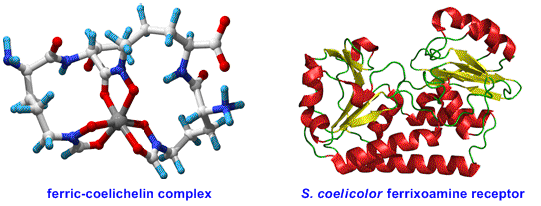
Research Interests
Research in the Challis / Alkhalaf group encompasses diverse aspects of the chemistry and biology of natural products, including genomics-driven discovery of new bioactive metabolites, elucidation of novel pathways for natural product biosynthesis, mechanistic enzymology of unusual biosynthetic enzymes, biosynthetic engineering approaches to the production of novel natural product derivatives, the total synthesis of the natural products we discover, and elucidation of the biological functions of natural products.
Genomics-driven natural product discovery
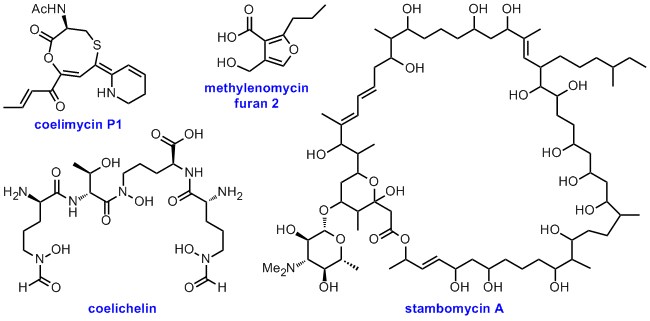
We are pioneers of the exploitation of microbial genomics for the discovery of novel bioactive metabolites. This has resulted in the discovery of numerous novel natural products, including the nonribosomal peptide iron-chelator coelichelin, the methylenomycin furan signalling molecules, the stambomycin complex of macrolide anticancer antibiotics, and the unusual polyketide alkaloid coelimycin P1. We have developed several important tools for analysing "cryptic" biosynthetic gene clusters uncovered by genomics and for identifying the metabolic products of such gene clusters, including methods for activating the expression of gene clusters that are expressed poorly or not at all under laboratory growth conditions. Our current focus is the development of efficient genomics- and biotechnology-driven natural product discovery pipelines that are suitable for implementation within industrial research and development programmes.
Representative publications: Chem. Sci. 2012, 3, 2716-2720; Proc. Natl. Acad. Sci. USA, 2011, 108, 6258-6263; Proc. Natl. Acad. Sci. USA 2008, 105, 17510-17515; Nat. Chem. Biol. 2005, 1, 265-269; Nature, 2002, 417, 141-147.
Elucidation of biosynthetic pathways
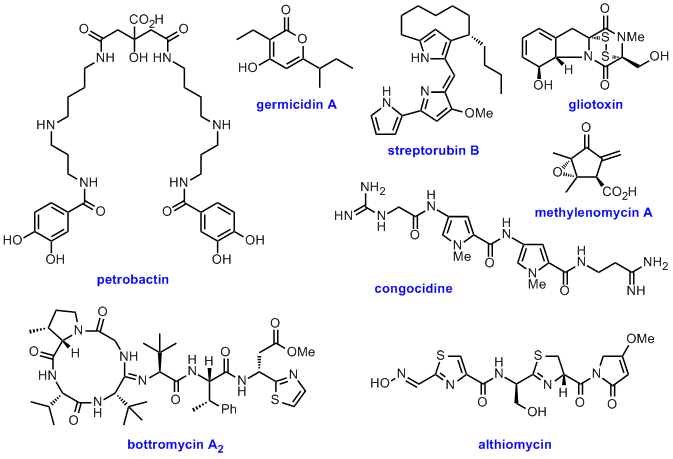
A highly interdisciplinary approach that encompasses molecular genetic manipulation (deletion and heterologous expression of biosynthetic genes), chemical synthesis of putative biosynthetic intermediates, characterisation of purified remcombinant biosynthetic enzymes, incorporation of labelled precursors, and a variety of analytical chemistry techniques, is employed to establish the pathways for assembly of a diverse array of bioactive microbial metabolites. Examples include: petrobactin, germicidin A, streptorubin B, gliotoxin, methylenomycin A, congocidine and the bottromycins. The results of such studies provide the fundametal knowledge required to develop engineered biosynthesis strategies for the production of novel natural product analogues.
Representative publications: Angew. Chem. Int. Ed. 2012, 51, 7454-7458; Chem. Sci., 2012, 3, 3522-3525; PLoS ONE, 2012, 7, e44673; Chem. Biol. 2011, 18, 542-552; Chem. Commun. 2008, 4034-4036; Chem. Biol. 2008, 15, 137-148; J. Am. Chem. Soc., 2007, 129, 8416-8417; J. Am. Chem. Soc. 2006, 128, 14754-14755; Chem. Commun. 2006, 3981-3983; ChemBioChem, 2005, 6, 2166-2170.
Mechanistic studies of unusual biosynthetic enzymes
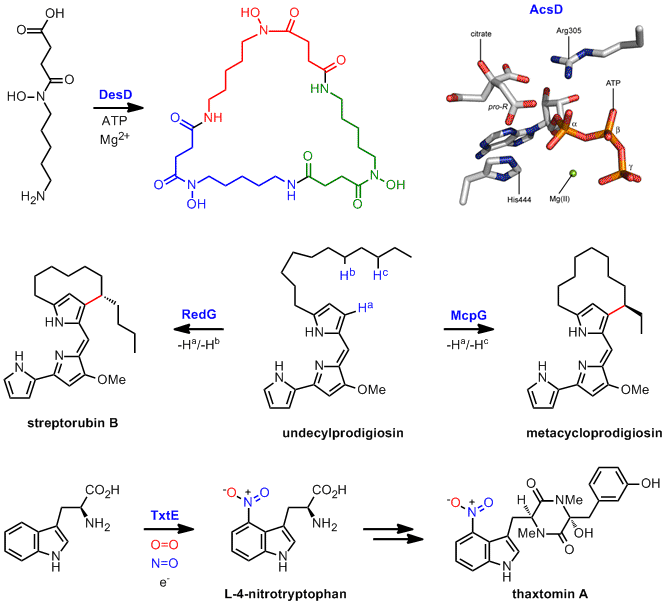
The catalytic mechanisms of enzymes responsible for highly unusual reactions in the biosynthesis of several important metabolites is being investigated utilising a combination of chemically- and enzymatically-synthesised substrates and substrate analogues (designed as mechanistic probes), X-ray crystallography, site-directed mutagenesis, and a variety of kinetic methods. Enzymes of interest include: DesD (desferrioxamine biosynthesis) and related ATP-dependent oligomerisation-macrocyclisation biocatalysts, AcsD (achromobactin biosynthesis) and analogous citrate desymmetrising enzymes, the Rieske oxygenase-like stereo- and regiodivergent oxidative carbocyclases RedG/McpG (streptorubin/metacycloprodigiosin biosynthesis), and the unique indole-nitrating cytochrome P450 TxtE (thaxtomin A biosynthesis).
Representative publications: Nat. Chem. Biol. 2012, 8, 814-816; Nat. Chem. 2011, 3, 388-392; Chem. Commun. 2009, 1389-1391; Nat. Chem. Biol. 2009, 5, 174-182; Chem. Commun. 2008, 5119-5121; J. Am. Chem. Soc. 2008, 130, 10458-10459; Nat. Chem. Biol. 2007, 3, 652-656.
Biosynthetic engineering
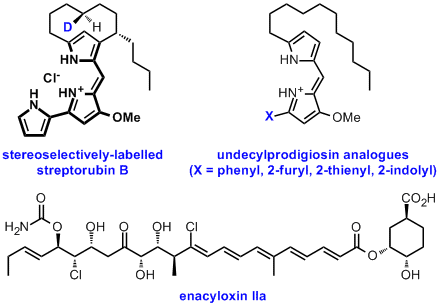
Several different approaches for manipulating the biosynthetic pathways to "high value" natural products with potential pharmaceutical and agrochemical applications, as well as for the de novo construction of novel biosynthetic pathways for the production of biofuels and platform chemicals, are being developed and explored. For example, using a mutasynthesis approach a stereoselectively deuterium-labelled streptorubin B analogue has been produced, resulting in elucidation of the absolute stereochemistry of the natural product, and several undecylprodigiosin analogues have been prepared. We are also developing approaches for manipulation of the enacyloxin IIa biosynthetic pathway, with the aim of producing more stable derivatives of this promising antibiotic.
Representative publications: J. Am. Chem. Soc. 2011, 133, 1793-1798; Chem. Biol. 2011, 18, 665-677; Chem. Commun. 2008, 1865-1867.
Biological functions of natural products
We are investigating the biological functions of a variety of metabolites, including iron-transporting siderophores (e.g. coelichelin and desferrioxamine E), signalling molecules that induce the production of antibiotics (e.g. the methylenomycin furans), and antimalarial alkaloids such as the prodiginines. Chemical synthesis of analogues is combined with a range of microbiological and biophysical techniques to identify the biological targets of such natural products and to define their structure-activity relationships.
Representative publications: J. Med. Chem. 2011, 54, 5296-5306; Biochemistry, 2010, 49, 8033-8042; J. Struct. Funct. Genomics, 2010, 11, 167-180; Proc. Natl. Acad. Sci. USA 2008, 105, 17510-17515; Microbiology, 2006, 152, 3355-3366.