Publications RSS
• A Scanning Electrochemical Cell Microscopy Platform for Ultra-sensitive Photo-electrochemical Imaging, Anal. Chem., 2015
Developing nanoscale structure-activity correlations is of major importance for the fundamental understanding and rational development of (photo)electrocatalysts. However, the low conversion efficiency of characteristic materials generates tiny photoelectrochemical currents at the submicron to nanoscale, in the fA range, which are challenging to detect and measure accurately. Here we report the coupling of scanning electrochemical cell microscopy (SECCM) with photo-illumination, to create a sub-micron spatial resolution cell that opens up high resolution structure-(photo)activity measurements. We demonstrate the capabilities of the technique as a tool for: (i) high spatial resolution (photo)activity mapping using an ionic liquid electrolyte at a thin film of TiO2 aggregates, commonly used as a photo-anode in dye sensitized solar cells (DSSCs); and (ii) in-situ (photo)activity measurements of an electropolymerized conjugated polymer on a transparent Au substrate in a controlled atmospheric environment. Quantitative data, including localized (photo)electrochemical transients and external quantum efficiency (EQE) are extracted, and prospects for further technique development and enhancement are outlined.
• Redox-Dependent Spatially Resolved Electrochemistry at Graphene and Graphite Step Edges, ACS Nano, 2015
The electrochemical (EC) behavior of mechanically exfoliated graphene and highly oriented pyrolytic graphite (HOPG) is studied at high spatial resolution in aqueous solutions using Ru(NH3)63+/2+ as a redox probe whose standard potential sits close to the intrinsic Fermi level of graphene and graphite. When scanning electrochemical cell microscopy (SECCM) data are coupled with that from complementary techniques (AFM, micro-Raman) applied to the same sample area, different time-dependent EC activity between the basal planes and step edges is revealed. In contrast, other redox couples (ferrocene derivatives) whose potential is further removed from the intrinsic Fermi level of graphene and graphite show uniform and high activity (close to diffusion-control). Macroscopic voltammetric measurements in different environments reveal that the time-dependent behavior after HOPG cleavage, peculiar to Ru(NH3)63+/2+, is not associated particularly with any surface contaminants but is reasonably attributed to the spontaneous delamination of the HOPG with time to create partially coupled graphene layers, further supported by conductive AFM measurements. This process has a major impact on the density of states of graphene and graphite edges, particularly at the intrinsic Fermi level to which Ru(NH3)63+/2+ is most sensitive. Through the use of an improved voltammetric mode of SECCM, we produce movies of potential-resolved and spatially resolved HOPG activity, revealing how enhanced activity at step edges is a subtle effect for Ru(NH3)63+/2+. These latter studies allow us to propose a microscopic model to interpret the EC response of graphene (basal plane and edges) and aged HOPG considering the nontrivial electronic band structure.
• Quad-Barrel Multifunctional Electrochemical and Ion Conductance Probe for Voltammetric Analysis and Imaging, Anal. Chem., 2015
The fabrication and use of a multifunctional electrochemical probe incorporating two independent carbon working electrodes and two electrolyte-filled barrels, equipped with quasi-reference counter electrodes (QRCEs), in the end of a tapered micron-scale pipet is described. This ‘quad-probe’ (4-channel probe) was fabricated by depositing carbon pyrolytically into two diagonally opposite barrels of a laser-pulled quartz quadruple-barrelled pipet. After filling the open channels with electrolyte solution, a meniscus forms at the end of the probe and covers the two working electrodes. The two carbon electrodes can be used to drive local electrochemical reactions within the meniscus while a bias between the QRCEs in the electrolyte channels provides an ion conductance signal that is used to control and position the meniscus on a surface of interest. When brought into contact with a surface, localized high resolution amperometric imaging can be achieved with the two carbon working electrodes with a spatial resolution defined by the meniscus contact area. The substrate can be an insulating material or (semi)conductor, but herein we focus mainly on conducting substrates that can be connected as a third working electrode. Studies using both aqueous and ionic liquid electrolytes in the probe, together with gold and individual single walled carbon nanotube samples, demonstrate the utility of the technique. Substrate generation-dual tip collection measurements are shown to be characterized by high collection efficiencies (approaching 100%). This hybrid configuration of scanning electrochemical microscopy (SECM) and scanning electrochemical cell microscopy (SECCM) should be powerful for future applications in electrode mapping, as well as in studies of insulating materials as demonstrated by transient spot redox-titration measurements at an electrostatically charged Teflon surface and at a pristine calcite surface, where a functionalized probe is used to follow the immediate pH change due to dissolution.
• A practical guide to using boron doped diamond in electrochemical research, Phys. Chem. Chem. Phys., 2015
Conducting, boron doped diamond (BDD), in addition to its superior material properties, offers several notable attributes to the electrochemist making it an intriguing material for electrochemical research. These include the widest solvent window of all electrode materials; low background and capacitive currents; reduced fouling compared to other electrodes and; the ability to withstand extreme potentials, corrosive and high temperature/pressure environments. However, BDD is not your typical electrode material, it is a semi-conductor doped degenerately with boron to present semi-metallic characteristics. Input from materials scientists, chemists and physicists has been required to aid understanding of how to work with this material from an electrochemical viewpoint and improve electrode quality. Importantly, depending on how the BDD has been grown and then subsequently treated, prior to electrochemical measurement, the resulting material properties can vary quite significantly from one electrode to the next. This likely explains the variability seen by different researchers working on the same experimental systems. The aim of this “protocols” article is not to provide a state-of-the-art review of diamond electrochemistry, suitable references are provided to the interested reader, but instead serves as a reference point for any researcher wishing to commence work with diamond electrodes and interpret electrochemical data. It provides information on how best to characterise the material properties of the electrode before use and outlines the interplay between boron dopant density, non-diamond-carbon content, grain morphology, surface chemistry and redox couple identity. All should ideally be considered when interpretating electrochemical data arising from the diamond electrode. This will aid the reader in making meaningful comparisons between data obtained by different researchers using different diamond electrodes. The guide also aims to help educate the researcher in choosing which form of BDD is best suited to their research application.
• Concluding remarks: there's nowt so queer as carbon electrodes, Faraday Discuss., 2014
This contribution provides a personal overview and summary of Faraday Discussion 172 on “Carbon in Electrochemistry”, covering some of the key points made at the meeting within the broader context of other recent developments on carbon materials for electrochemical applications. Although carbon electrodes have a long history of use in electrochemistry, methods and techniques are only just becoming available that can test long-established models and identify key features for further exploration. This Discussion has highlighted the need for a better understanding of the impact of surface structure, defects, local density of electronic states, and surface functionality and contamination, in order to advance fundamental knowledge of various electrochemical processes and phenomena at carbon electrodes. These developments cut across important materials such as graphene, carbon nanotubes, conducting diamond and high surface area carbon materials. With more detailed pictures of structural and electronic controls of electrochemistry at carbon electrodes (and electrodes generally), will come rational advances in various technological applications, from sensors to energy technology (particularly batteries, supercapacitors and fuel cells), that have been well-illustrated at this Discussion
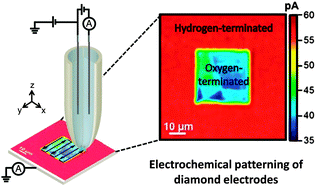
• Electrochemical "read-write" microscale patterning of boron doped diamond electrodes, Chem. Comm., 2014
Scanning electrochemical cell microscopy is utilised as a read–write pipette-based probe to both electrochemically modify the local surface chemistry of boron doped diamond and “read” the resulting modification, at the micron scale. In this specific application, localised electrochemical oxidation results in conversion of the H-terminated surface to –O, electrochemically visualised by monitoring the current change for reduction of Ru(NH3)63+. This methodology, in general, provides a platform for read–write analysis of electrodes, opening up new analytical avenues, particularly as the pipette can be viewed as a microfluidic device.