Research
Current research
Research in the Habershon group focuses on development and application of new methods for modelling chemical dynamics in complex many-particle systems. Our interests span catalysis, nanoparticle, photochemistry and reaction rate evaluation.
|
|
Predicting reaction mechanisms using random walks in chemical space. We have developed graph-driven sampling methods to enable searches for reaction paths in complex chemical systems [See: J. Chem. Phys., 143, 094106 (2015) and J. Chem. Theory Comput., 12, 1786 (2016)]. Our approach allows us to automatically sample reaction paths connecting different conformations and chemical isomers. We have recently expanded our approach to enable determination of double-ended reaction-mechanisms, providing a route to testing mechanistic hypothesis in a direct and automated fashion [See: J. Phys. Chem. A, 123, 3407-3417 (2019) and Cat. Sci. Tech., 9, 6357-6369 (2019)]. In the initial application of this graph-based sampling approach, we've shown that the main steps in the accepted mechanism of cobalt-catalyzed ethene hydroformylation are captured; current work is focussed on using this approach to study combustion processes, homogeneous and heterogeneous catalysis, atmospheric chemistry and astrochemistry. |
|
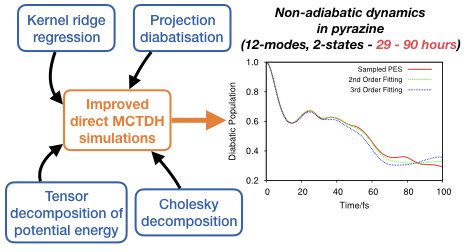
New methods for on-the-fly quantum dynamics. We have shown how one can combine machine-learningtools, such as kernel ridge regression, with accurate wavefunction propagation methods like MCTDH [Chem. Phys. Letters, 683, 228 (2017), and J. Chem. Phys., 148 (13), 134116 (2018)]. Recently, we have shown how the same ML-based quantum dynamics scheme can be used in combination with the variational multi configurational Gaussian method [J. Chem. Phys., 150, 041101 (2019)] and to study spin-forbidden processes [J. Phys. Chem. A, 124, 9299-9313 (2020)]. |
 |
 |
Network organization in photosynthetic complexes. In another recent paper, we have used simple quantum dynamics simulations of energy transport, combined with network-based analysis of Frenkel exciton Hamiltonians, to investigate the influence of network organization on photosynthetic efficiency. Our results revealed an interesting aspect of the Fenna-Matthews-Olson phototsynthetic complex: around 50% of the energy transport network can be removed with little impact on energy transport efficiency. The full paper can be found at J. Chem. Phys., 143, 105101 (2015). |