Macpherson Group Research

Led by Professor Julie Macpherson, our research utilises the exceptional properties of laboratory grown Boron Doped Diamond (BDD) for a variety of electrochemical applications.
Explore the sections below to find out more:
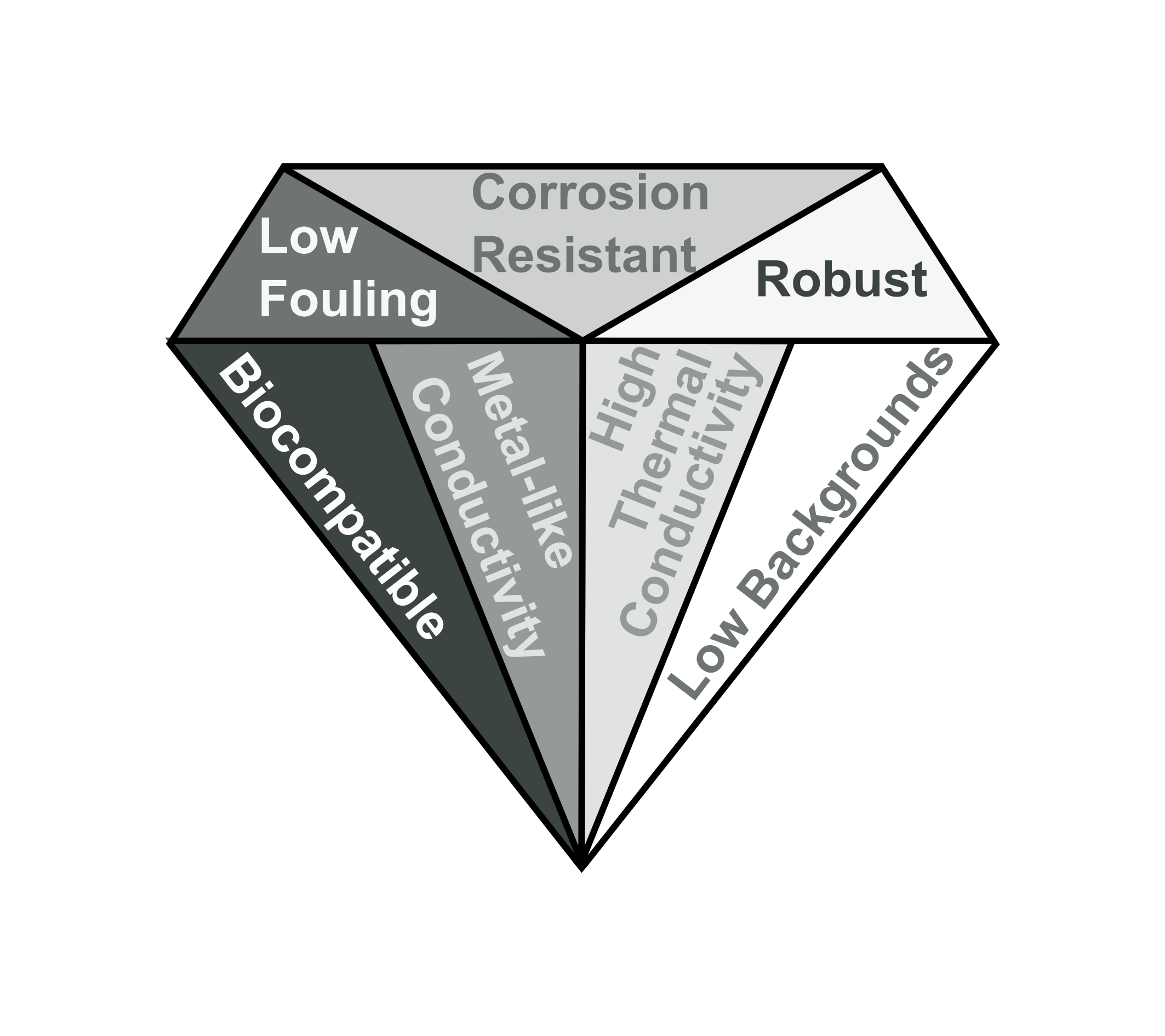
What is Boron Doped Diamond (BDD)
Diamond is an exceptional material as a result of its sp3 hybridisation, which results in a number of advantageous properties such as high thermal conductivity, extremely high electrical resistivity, and obviously hardness! Lab-grown diamonds can be produced relatively inexpensively using a technique known as chemical vapour deposition (CVD). In CVD the diamond is deposited from a plasma, which contains CH4 and H2.
If boron atoms are present in the plasma they can be intergrated into the diamond lattice during replacing a carbon atom. If enough carbon atoms are replaced with boron (around 1 in 1000) it becomes electrically conductive, exhibiting a number of beneficial properties as an electrode material; these include wide solvent window, low background currents, reduced fouling, biocompatibility, and corrosion resistance. In our laboratory we endeavour to exploit many of these aforementioned material properties in our research. Most of the BDD we use is cut from high-quality freestanding chemical vapour deposition (CVD) grown wafers.
See: Macpherson, Phys. Chem. Chem. Phys., 2015,17, 2935-2949
Biomedical Sensors
Using conducting diamond electrodes we are developing sensors for a wide range of biomedical applications.
On-skin Sensors in Partnership with the NHS: One key application is the minimally-invasive measurement of blood gases in neonatal babies. Continuous on skin monitoring removes the need for blood withdrawal and enables rapid clinical response to changing medical condition.
Tissue pH Sensing: Our sensors are also used to map the pH of gastrointestinal (GI) tissue, offering key insights into the effects of pharmaceutical interventions for GI distress.
See: Read et. al. ACS Sens. 2019, 4, 3, 756–763 and Rajan et. al. ACS Sens. 2020, 5, 9, 2858–2865


Low-cost Sensing and Instrumentation
We are working on methods to use low-cost and rapid manufacturing techniques (like 3D-Printing) to reduce costs and improve the accessibility of electrochemical tools and methods.
Citizen Science: We are developing low cost and reusable sensors for use by the general public to enable public participation in UK wide water sampling activities e.g. monitoring the health of the UK’s river systems.
Low-cost Instrumentation for Chemical Education: We are using 3D printing to produce a variety of electrode configurations for use in research and teaching.
New Materials for 3D Printed Electrodes: Development of new conductive materials which can be used for 3D printed electrodes.
Catalysis
We are working on single atom and nanoparticle electrochemisty for energy applications, often involving combining multiple techniques, such as Transmission Electron Microscopy (TEM), Scanning Electrochemical Cell Microscopy (SECCM), and bulk electrochemistry.
BDD TEM Grids: We can fabricate TEM grids from BDD, which are superior to the coventional carbon grids, due to their stability, low electrochemical backgrounds, high thermal conductivity, low scattering. these grids enable identical location correlated TEM and electrochemical experiment to track individual particles on the atomic scale.
Green Energy: We are using TEM to study BDD as an electrocatalyst support for Pt in PEM fuel cells and Ir/IrOx in electrolysers for green hydrogen production.
Single Atom Catalysis: Using SECCM and TEM we can study the electrochemisty of single atoms, and discover how single atoms differ from their nanoparticle counterparts in reaction rate and products.


Enviromental Remediation
We are using electrochemical advanced oxidation technologies with BDD electrodes to tackle pressing environmental challenges.
PFAS Destruction: Forever chemicals e.g. perfluoroalkyl substances (PFAS), are not naturally broken down by natural processes and have been linked to a number of health concerns in humans. However, it is thought that these chemicals can be destroyed by electrochemical advanced oxidation on BDD electrodes.
Electrode Corrosion Tracking: BDD electrodes are more expensive than other electrode materials, so it is important that they last longer in the field to offset this cost. However, measurement of electrode corrosion in these systems is time consuming and difficult. To overcome this, we have developed rapid methods to measure the extent of corrosion in the lab, to give an estimate of electrode lifetimes in the field.
See: Tully et. al. ACS Meas. Sci. Au 2024, XXXX, XXX, XXX-XXX
Electrosynthesis
For some molecules electrosynthesis can be a green alternative to conventional chemical synthesis, in the group we explore BDD as an electrode for a number of electrosynthesis applications.
Electrochemical Ozone Production: Ozone can be produced directly from water, as a product of the water oxidation reaction, however this requires an electrode that disfavours oxygen evolution and can tolerate high current denisties.
Hydrogen Peroxide Production: Hydrogen Peroxide is a commodity chemical with applications in a wide range of industries. the majority of Hydrogen peroxide is produced by the anthraquinone process on tons scales, however this process utilises a carcinogenic quinone as its catalyst, and produces organic solvent waste. Electrochemistry offers a solution to these problems as it can produce hydrogen peroxide directly from aqueous electrolytes.
.

Advanced Fabrication
Whilst extreme hardness is one of diamonds unique properties, it also presents one of the major challenges when fabricating devices from this material. The overcome these challenges we use a number of specialist fabrication tools, such as Laser Micromachining, Reactive Ion Etching, Physical Vapour Deposition, and Electrochemical Etching.
See: Tully et. al. Carbon, Volume 185, 2021, and Ayres et. al. Anal. Chem. 2016, 88, 1, 974–980
