Bacteriophages and Phage Therapy
When Alexander Fleming discovered penicillin in 1928, the first modern antibiotic, he revolutionised the way bacterial infections were treated. However, antibiotics are a double-edged sword: without them, diseases such as pneumonia, chlamydia, and urethritis just to name a few would persists, yet with growing use of antibiotics comes the growing issue of antibiotic resistance, among others. And with that, enter phage therapy.
Phage therapy refers to the use of viruses that specifically infect and kill bacteria (known as bacteriophages or phages for short) to treat bacterial infections. As bacteriophages do not infect humans, they have potential to be used in medical treatment of infections. Phage therapy is by no means a new concept; the first use of phage therapy date back to around 100 years ago, but its development was halted by the introduction of antibiotics. However, phage therapy is seeing the light of day once more.
Earlier this year, a teenage cystic fibrosis, Isabelle, was suffering from a bacterial infection with and was given less than a 1% chance of survival. Having had a lung transplant and a compromised immune system due to immunosuppressant medication, Isabelle’s options were limited. But, thanks to her mother’s research into alternative treatment options, doctors decided to attempt phage therapy.
Isabelle was injected with a “cocktail” of three phages (two of which had been genetically modified to make them more effective) twice each day, which was also applied onto her skin lesions. Isabelle’s lesions soon began to heal, and while she is not completely cured of the infection, it is under control. She must continue to receive daily injections of the viral cocktail, and is currently awaiting the addition of another phage to the mix in an attempt to eradicate the bacteria from her body completely.
So, what exactly are phages and how do they work?
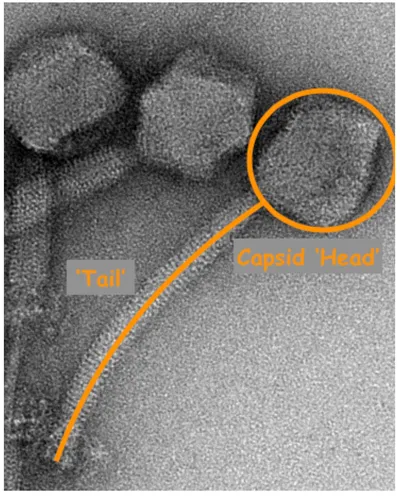
The word ‘bacteriophage’ literally translates from Greek to ‘bacteria eater’. Phages are present everywhere in nature, and infect specific species of bacteria; some phages can also infect archaea. Phages, like all viruses, contain genetic information in the form of nucleic acids (either RNA or DNA) enclosed in a capsid (i.e. protein envelope). This makes up the ‘head’ of bacteriophage, while the ‘tail’ is made up of proteins with a receptor at the end which helps the virus attach to the surface of bacteria. Some phages don’t have a ‘tail’, and some even exist as long filaments.
Bacteriophages have two life cycles by which they replicate: lysogenic and lytic. In a lysogenic cycle, the viral genes are inserted into the bacterial chromosome and undergo transcription and translation – in this life cycle, infected bacteria do not die. On the other hand, in the lytic cycle, the virus replicates within the host cell which is then killed when replicated viruses burst (or lyse) the cell to escape.
Because phages only attack specific bacteria, they can be very useful in treating some infections. For example, scientists have isolated phages which infect superbugs like Clostridium difficile which causes diarrhoea and colitis. Treating this infection with antibiotics would result in a disruption of the gut microbiota, making the specificity of bacteriophage therapy a particularly useful tool.
On top of this, it is far more difficult for bacteria to develop resistance to phages. This is because phages attack bacteria and kill by bursting the cell wall and membrane, whereas antibiotic disrupt a specific process that occurs within the cell. What’s more, many bacteria can form biofilms which protects them from antibiotics, but some phages have evolved to break down this biofilm and kill the bacteria.
While phage therapy is not approved or regulated in the UK and the West, a new clinical trial has been launched at the start of 2019 in the US, meaning that phage therapy may potentially eradicate our need for antibiotics.
