Frequently Asked Questions (FAQs)
UPDATES to FAQs:
02.03.2023- We have provided additional guidance on recording periods of ventilation and extubation (found under participant eligibility)
11.01.2023- We have provided additional guidance on what are the exercise contraindications that would exclude a patient from joining iRehab (found under participant eligibility)
07.12.2023- We have provided additional guidance on whether participants should be included/excluded based on whether they are receiving condition specific evidence based rehabilitation
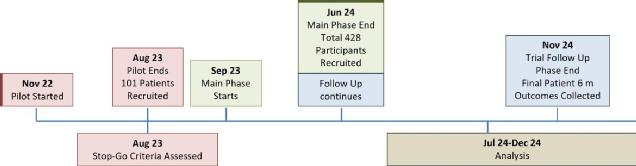
Study Time Line
Site Eligibility
• Usually follow up services would not exclude a site from participating. It depends on what the referral pathway is, and whether it is your standard or routine practice for all patients to be referred to onwards to a rehabilitation programme or not.
• If your follow up care consists of a single contact or phone call from a healthcare practitioner (e.g., therapist/nurse etc) once patient is discharged home, your site is eligible to take part in iRehab.
• If your follow up care consists of one or more staff with services/triage that does not include structured rehabilitation your site is eligible to take part in iRehab
• If your standard care is a rehabilitation programme that consists of more than one contact or phone call, e.g., offering weekly support or therapy (talking therapy, physiotherapy, occupational therapy, home visits or similar) or weekly/regular exercise sessions, or weekly/regular support group or education sessions for a period after a patient is discharged home, then your site will not usually be eligible for iRehab. This is because it is likely too similar to what we want to investigate.
• If your standard care differs from these examples, then collect details and please get in touch with us and we would be happy to discuss this further.
This means a programme designed to provide regular support and/or rehabilitation to people after they get home from hospital, and which is delivered for a defined period of time (6 weeks for iRehab). In iRehab, patients randomised to the intervention will be contacted by the intervention team every week for six weeks to see how they are coping with their symptoms; participants will also be asked to take part in weekly online exercise classes and online weekly support groups. The exercises the participants receive are also ‘structured’ using a set menu of gentle exercises to follow. Exercises will then progress, depending on ability, over the following weeks. If you are unsure whether your usual care is similar to this programme, then please get in touch and we would be happy to discuss this further. (Also see FAQ "What does the physical Intervention involve /how long are the appointments?").
About Site Staff
No, the sponsor has approved that non-medical PIs, e.g., a physiotherapist, nurse, or any other trained healthcare professional could be the PI. All PIs and co-PIs will be expected to have completed GCP training and will need to provide an up-to-date GCP certificate and CV in line with local SOPs. We encourage involvement from allied health professionals.
Yes, however sites will need to nominate the lead PI for documentation purposes. The lead PI can then delegate responsibility to the co-PI as recorded by the site delegation log.
Consent to join the trial will usually be taken over the telephone or by video call, once the participant has returned home and this will be actioned by an appropriately trained member of the team listed on the delegation log.
To consent participants, the delegated individual must have completed their GCP training in line with local SOPs.
Any member of the team who has received appropriate training and has been delegated the responsibility by the PI can confirm eligibility.
Sites have the option of identifying an ‘iRehab champion’. This is someone who would be a key team member, who will serve as a liaison between the trial, patients, and intervention teams. This could be any member of staff with the relevant experience to enable them to undertake the activities described below.
1. Be an advocate and an important contact for the trial helping with promotion of the iRehab trial.
2. Be the main point of contact for the intervention team, provide information handover and help with resolving intervention-related queries relevant to their local site.
3. Assist with understanding specific cultures within each site
4. Link with participants post discharge to check their communication details and technology access are available and working (e.g., phone, email address, Teams)
5. Link with the intervention team to identify services for onward referral if this is needed at the end of the intervention period.
6. Help the team and the local PI with data queries at site.
7. (If needed) help the local team follow up potential participants to obtain consent.
8. Agree to liaise with trial team to resolve issues, where needed.
9. Undertake any other reasonable activity to support the conduct of the study at the site.
Please be aware that this role description is not exhaustive and will be regularly evaluated by the TMG and may evolve during the trial. We may adapt this list of duties after the pilot phase and the role of ‘Champion’ has been tested in practice.
Yes, there is an agreed amount of funding for the iRehab champion. This is outlined in the OID and mNCA documentation, until the end of recruitment. The role will be reviewed periodically and invoiced quarterly.
Study Information
Yes. The iRehab trial CPMS number is 53647.
The suggested total recruitment per site is about 15 participants per site over 18 months. If you have any questions, please contact the iRehab team.
The pilot will commence October 2022-June 2023. Pilot phase is shaded in the image above.
428 participants – this includes the 101 recruited to the pilot study.
Yes. The pilot phase will last 9 months and will recruit the first 101 participants. During the pilot, we will test trial processes and procedures. The data collected will be included in the main trial analysis. The pilot phase is planned to transition seamlessly into the main trial (after review and approval). The pilot phase is important to test our processes and procedures.
We plan to open at least 30 across the UK.
Study Processes
Patients can be identified from ICU databases whilst they are still in hospital and assessed for eligibility at this point e.g., if they have been on mechanical ventilation for longer than 48 hours. Once discharged from hospital, recruitment and consent will usually be via telephone by the hospital research team. Potential participants could also be recruited from ICU follow up clinics if patients are still within 12 weeks of getting discharged and no further rehabilitation is planned at your site that would align to the study timelines.
Site staff will need to complete some screening information and a small amount of information (contact details, demographics and details of admission) on each participant and will be required to submit this electronically via the iRehab database.
Site staff may also be required to complete readmission, withdrawal, notification of death, adverse event, or serious adverse event forms as these arise.
No, all patient facing questionnaires will be sent to participants, and managed by the WCTU Trial Management Team.
WCTU Trial Management Team
No, the outcome assessor at WCTU completes the sit to stand test with the patient pre randomisation via video conferencing or telephone.
Sites will not receive ETCs (as they are not involved with delivering any of the intervention). The intervention/treatment for this trial is being delivered by a central team based at Ulster University and Belfast Health and Social Care Trust.
The OID and mNCA sets out the research costs the iRehab will be providing to sites to facilitate the trial and covers the funding model per randomised patient and for the research champion. Please see the following documents for more detail:
• Organisation_Information_Document_NonCommercial_iRehabV1.0 30.03.2022, Page 5 Section 15
• mNCA_iRehab_v1.0 30.03.2022, Schedule 3 Page 49
Yes, the iRehab team at WCTU, will approve recruitment in through the CPMS.
We currently do not permit sites to complete EQ5D CRF at registration and consent. The patient will receive the link to the questionnaires as soon as they are registered on the database, however as this is a remote intervention it is a fundamental step to ensure that the patient will be able to complete the intervention/data collection remotely. Our outcome assessor’s role is to ensure the patient is capable of partaking in the study and completion of the patient information will form part of this process- this also will ensure consistency and continuity when collecting data relating to the trial outcomes.
The only time we may ask sites to aid a patient in completing the EQ5D is if the participant is an inpatient at the 8-week outcome data collection time point. This will be requested and discussed with site staff on a case-by-case basis.
There is no assent mechanism for consent to the iRehab trial. Patients must have the capacity for informed consent to the trial, however we do understand that this patient population may require additional support. We fully support and recommend that, where appropriate, you engage with the patient’s family to discuss the trial with them. This is no different from any other research study where it is recommended that prospective participants can discuss with close family members or their GP if they wish.
The iRehab intervention is targeted and progressive based on the individuals’ requirements, physical abilities, and comorbidities. Once a patient has been randomised to the intervention, they will have a 1-to-1 remote assessment with a trained healthcare practitioner for a comprehensive needs assessment. They will be prescribed a treatment plan according to their symptoms (individualised). The trained intervention team will select appropriate activities from an exercise ‘menu’. This menu comprises different types of exercises and intensities, which will be tailored to the individual over their 6 weeks on the intervention. The trained healthcare team have experience of prescribing rehabilitation/exercise to individuals with a range of health conditions.
It is under the responsibility of WCTU to source and provide a mobile phone for a participant to use during their duration of the trial if the patient does not have access to a phone (home or mobile). This will be assessed on a case-by-case basis and will require review and approval from the WCTU Trial management team. If you have any questions about this, please contact the iRehab team using the contact details provided at the end of the document.
Upon finalisation of the database, CRFs will be shared as part of the ISF. These will be shared with sites as soon as possible, and the ISF shared upon receipt of a localised mNCA agreement, unless otherwise agreed.
Yes, its imperative for trial procedures to ensure there is adequate documentation to satisfy that all parties are aware of the participants enrolment into the iRehab trial.
Providing we have confirmed that the follow up clinic you offer to patients doesn’t replicate the content of the iRehab intervention, you can still refer patients to other services within the NHS should this be required and as per your usual care. If the intervention team identify a requirement for an onward referral to provide additional support services or treatments, they may contact the site research champion to discuss whether the site can facilitate this as per local standard care.
Please see below a checklist for site opening:
Step 1) Receive EOI
Step 2) Eligibility/Site Feasibility meeting
Step 3) Site receive LIP back inc NSA- addition of trial site
Step 4) Send WCTU localised mNCA for Sponsor sign off
Step 5) mNCA returned to site for final signatures
Step 6) ISF sent to site and SIV arranged
Step 7) Site to send WCTU copies of all localised docs
Step 8) SIV commencement and Delegation log/training log sign off
Step 9) C&C issued by site
Step 10) WCTU Greenlighting, and database access provided
Participant Eligibility
The physical activity/exercise component in iRehab will be tailored according to an individual’s ability, symptoms, and comorbidities. There are very few conditions where exercise/physical activity is not recommended. To help healthcare professionals recognise individuals who should not be considered for iRehab, there are recognised absolute contraindications to exercise/exercise testing in accordance with the American College of Sports Medicine [2017]:
• A recent significant change in the resting electrocardiogram suggesting significant ischemia, recent myocardial infarction (within 2 d), or other acute cardiac event
• Unstable angina
• Uncontrolled cardiac dysrhythmias causing symptoms or hemodynamic compromise
• Symptomatic severe aortic stenosis
• Uncontrolled symptomatic heart failure
• Acute pulmonary embolus or pulmonary infarction
• Acute myocarditis or pericarditis
• Suspected or known dissecting aneurysm
• Acute systemic infection, accompanied by fever, body aches, or swollen lymph glands
Rehabilitation is mostly safe, feasible and acceptable for people following surgery and can be beneficial for many other chronic conditions. If there are any conditions you are unsure about or would like further advice on, these should be discussed with the PI and/or iRehab study team.
Reference: American College of Sports Medicine (2017) ACSM’s Health-Related Physical Fitness Assessment. 5th Edition. Lippincott Williams and Wilkins. United States. .
As per the eligibility criteria, patients are only eligible for the study when they have been discharged home. Therefore, patients can only be consented to the study when they have been discharged home from your care. If the participant is awaiting collection from hospital transport/relatives and would like to consent to the study, then this is possible, but only in cases where hospital discharge is confirmed. Please remember, that in line with good clinical practice you must ensure that your patients have had time to read and absorb the information and ask any questions they may have. Consenting the patient remotely via video link or telephone will also indicate if they have the appropriate facilities to be able to take part in the remote rehabilitation, allowing both site staff and the team at WCTU to ensure accessibility for anyone who wishes to take part. As a reminder, all intervention delivery will be done remotely (by phone or by video).
Record the most recent period of eligible ventilation (i.e. >=48 hours). We do not require a cumulative record of time on ventilation.
Cardiac and Pulmonary rehab (when delivered as per evidenced based guidelines to include structured exercise/education/peer and psychosocial support/lifestyle Mx) would be structured rehabilitation similar to iRehab, and we would prefer to exclude these patients. In general, if a specific condition pathway is available to participants, evidence-based practice should be followed.
Exceptions may be where the patient is not being referred to such service e.g. previously attended or the programme is not running.
Decision: If there is an existing evidence based pathway that the patient is going to attend, they should usually not be included. If there is not an established pathway or if not appropriate for the patient then the patient could be included. Similarly if the pathway is completely different to the iRehab intervention then the patient could be included.

Enquiries:
Please direct all enquiries to the iRehab team;
Kerry Raynes: Trial Manager
Louisa Edwards, Trial Coordinator
Katherine Jones, Research Fellow
Email: iRehab@warwick.ac.uk
Tel: 02476151367
Feasibility QuestionnaireLink opens in a new window
FAQs Link opens in a new window
