Trial Documentation
On this page you will find all relevant documents and resources for setting up and conducting the trial at site.
To help you with this, please find a version control log listing all of our documents here. If you wish to print these documents to create a paper ISF, please find an index here.
Thank you to all of our sites for your hard work so far. Please find a certificate of appreciation here.
Protocol
Participants Documents
Main Ethics
Amendments
Substantial Amendments
Non-Substantial Amendments
Documents for Site Greenlight
Data Collection
Participant Safety
Useful Documents/Guides
Working Instructions
Protocol
Participants Documents
Consent form
Patient Information Leaflet
Patient Invitation Flyer
Patient Invitation Letter
Baseline Questionnaire Form
3 Month Questionnaire Form
6 Month Questionnaire Form
12 Month Questionnaire Form
Translated Documents
Main Ethics
Organisation Information Document
Amendments
Substantial Amendments
-
Substantial amendment #1
- REC Approval
- Updates to documents:
- Participant Follow-up CLMHD Letter V1.0 26Oct2020
- Participant Workbook Letter V1.0
- Participant Consent Form v3.0 29Oct2020
- Participant Interview Consent Form V3.0 04Dec2020,
- Participant Information Sheet v3.0 29Oct2020,
- Participant Interview PIS V3.0 27Oct2020,
- Practitioner Interview PIS V3.0 29Oct2020,
- Protocol v3.0 20201029.
Non-Substantial Amendments
-
Non-substantial amendment #1
back to top
Documents for Site Greenlight
Site Training Slides
CRN Letter Site Targets
Validated SoECAT UHCW Activities
back to top
Data Collection
Baseline Form
Non-compliance Form
back to top
Participant Safety
back to top
Useful Documents/Guides
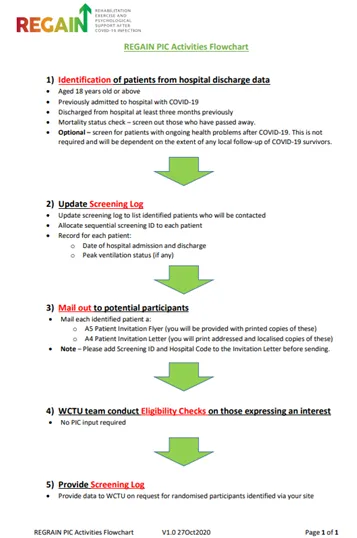 t
t
back to top
Working Instructions
SAE Handling Process Working Instruction
