Solving the puzzle of polymers binding to ice for Cryopreservation
· Cryoprotectants are used to protect biological material during frozen storage
· They have to be removed when defrosting, and how much to use and how exactly they inhibit ice recrystallisation is poorly understood
· The polymer poly(vinyl)alcohol (PVA) is arguably the most potent ice recrystallisation inhibitor and researchers from the University of Warwick have unravelled how exactly it works.
· This newly acquired knowledge base provides novel guidelines to design the next generation of cryoprotectants
When biological material (cells, blood, tissues) is frozen, cryoprotectants are used to prevent the damage associated with the formation of ice during the freezing process. New polymeric cryoprotectants are emerging, alongside the established cryoprotectants, but how exactly they manage to control ice formation and growth is still largely unknown. This is especially true for PVA, a deceptively simple synthetic polymer that interacts with ice by means of mechanisms that have now been revealed at the atomistic level thanks to researchers from the University of Warwick.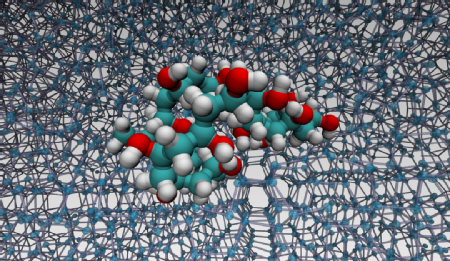
Cryoprotectants are crucial when freezing biological material to lessen the cellular damage involved with the formation of ice. Ice re-crystallization, that is the process by which larger ice crystals grow at the expense of smaller ones, is one of the major issues affecting the current cryopreservation protocols and it is still poorly understood. Researchers from the University of Warwick have investigated how a rather popular polymer with the potential to be used in cryopreservation binds to the growing ice crystals.
In the paper, ‘The atomistic details of the ice recrystallisation inhibition activity of PVA’, published in the journal Nature Communications, researchers from the University of Warwick have found that, contrary to the emerging consensus, shorter or longer polymeric chains of poly(vinyl)alcohol (PVA) all bind to ice.
Up to now, the community has been working under the assumption that short polymers do not bind strongly enough to the ice crystals, but in this work Dr. Sosso and co-workers have demonstrated that it is the subtle balance between these binding interactions and the effective volume occupied by the polymers at the interface with ice that determine their effectiveness in hindering ice re-crystallization.
This work brings together experimental measurements of ice recrystallization inhibition and computer simulations. The latter are invaluable tools to gain microscopic insight into processes such as the formation of ice, as they are able to see what is happening in very fast or very small processes which are hard to see via even the most advanced experimental techniques.
This work sheds new light onto the fundamental principles at the heart of ice re-crystallization, pinpointing design principles that can be directly harnessed to design the next generation of cryoprotectants. This achievement is a testament to the strength of what is affectionately known as ‘Team Ice’ at Warwick, an ever-growing collaborative network with the potential to make a huge impact on many aspects of ice formation, from atmospheric science to medicinal chemistry.
Fabienne Bachtiger, a PhD student working in the research group of Dr. Sosso (Department of Chemistry) who has spearheaded this work, explains:
“We have found that even rather short chains of PVA, containing just ten polymeric units, do bind to ice, and that small block co-polymers of PVA bind too. It is important for the experimental community to know this, as they have been working under different assumptions up to now. In fact, this means we can successfully use much smaller polymers than previously thought. This is crucial information to aid the development of new more active cryoprotectants.”
Dr. Gabriele Sosso, from the Department of Chemistry at the University of Warwick, who is leading a substantial computational effort to investigate the formation of ice in biological matter, points out that:
“With this contribution we have added a crucial piece to the puzzle of how exactly polymeric cryoprotectants interact with growing ice crystals. This is part of a larger body of computational and theoretical work that my group is pursuing with the intent to understand how cryoprotectants work at the molecular level, so as to identify designing principles that can be directly probed by our experimental colleagues. Warwick is the perfect place to further our understanding of ice, and this work showcases the impact of the very exciting collaboration between my research group and the Gibson Group.”
Professor Matthew Gibson, from the Department of Chemistry and Warwick Medical School at the University of Warwick adds: “Ice re-crystallization is a real challenge in cryobiology, leading to damage to cells but also in frozen foods or infrastructure. Understanding how even this ‘simple’ polymer works to control ice re-crystallization is a major step forward to discover new cryoprotectants, and ultimately to use them in the real world.”
ENDS
15 MARCH 2021
NOTES TO EDITORS
Paper available to view at: https://www.nature.com/articles/s41467-021-21717-z
High-res images available: https://warwick.ac.uk/services/communications/medialibrary/images/march_2021/sosso_ice_png.png
Caption: A molecular-level detail of the interaction between PVA and ice (from molecular dynamics simulations)
Credit: University of Warwick
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
