A-level Chemistry
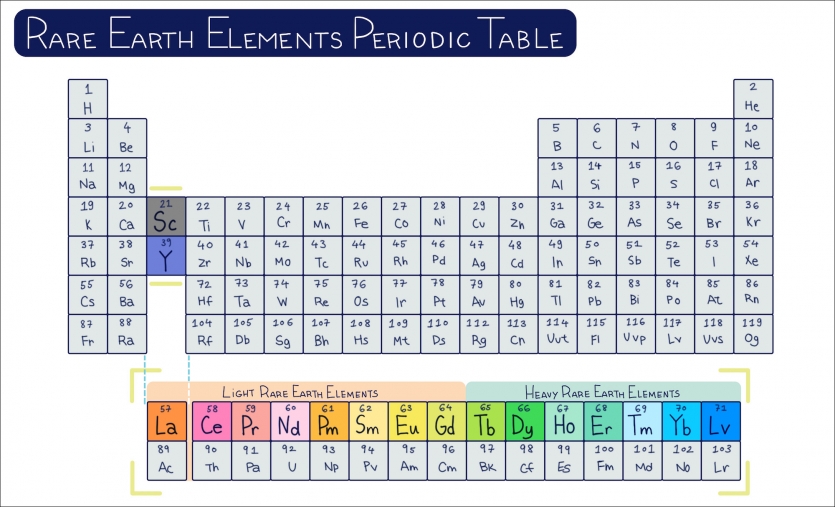
(The rare earth elements are sometimes also referred to as the lanthanides—referring specifically to the series of 15 elements running from lanthanum (La) to lutetium (Lu). [1]Link opens in a new window)
The rare earth elements took on a new scientific and then geopolitical importance with advances in atomic physics during the 20th century. The challenge of separating the rare earth elements from ore and from one another made it unclear just how many rare earth elements there might be. In 1913, the British physicist Henry Moseley determined there were 15 elements in the lanthanide series (atomic numbers 57 through 71) using X-ray spectroscopy [1]Link opens in a new window.
Rare earths acquired a new status in 1939, after Otto Hahn, Lise Meitner, and Fritz Strassmann discovered nuclear fission of uranium—an insight leading to the atomic bomb—and identified rare earth elements in fission products. In the United States the plan to build an atomic bomb, code-named the Manhattan Project, drew on the expertise of the leading American rare earth chemist, Frank Spedding, to solve a key problem. The rare earth elements were impurities that prevented a nuclear chain reaction by absorbing neutrons. The rare earths needed to be separated and removed in the process of purifying uranium. Out of Spedding’s wartime work was born the Ames Laboratory at Iowa State University, now the U.S. government’s premier rare earth elements research facility [1]Link opens in a new window.
Rare Earth Elements Education Resources for Chemistry
Rare Earth Elements
A whole new world for rare earths
Rare Earth Elements - A Brief Introduction
Rare earth elements
Separation of Rare Earth Elements by Charles James
A quiz about periodic table, easy to use and engage with class
Rare Earth Element (Overview)
A quiz about the periodic table and REEs
The History and Future of Rare Earth Elements
Rare Earth Elements

[Picture]Link opens in a new window
Here, we summuarized education resources for teaching Rare Earth Elements in the Chemistry course in the following examboards:
Assessment and Qualifcations Alliance (AQA)
Chemistry (7404, 7405)
Topic 3.1 Physical Chemistry
Topic 3.2 Inorganic Chemistry
EDEXCEL
Chemistry (2015)
Topic 1 Atomic Structure and the Periodic Table
Topic 4 Inorganic Chemistry and the Periodic Table
Oxford, Cambridge and RSA Examinations (OCR)
Chemistry A (H302, H432)
Chemistry B (H033, H433)
Module 2: Foundations in Chemistry
Module 3: Periodic Table and Energy
International Baccalaureate (IB)
Chemistry
Topic 2 Atomic Theory
Topic 3: Periodicity
Topic 4: Chemical bonding and structure
For Further Curriculum information, please visit the following examboard website.
Assessment and Qualifcations Alliance (AQA)
https://www.aqa.org.uk/
EDEXCEL
https://qualifications.pearson.com/en/home.html
Oxford, Cambridge and RSA Examinations (OCR)
https://ocr.org.uk/
International Baccalaureate (IB)
https://www.ibo.org/
