Circadian Clocks
My research in this area is very much concerned with understanding the design principles of circadian clocks. What are their roles and how is this reflected in their structure? I would add that since the clock is a dynamical system with a very complex molecular structure and since the role of the clock is clearly multifacited, this cannot be answered without the use of mathematics. My collaborators are listed here.
The circadian clock adapts organisms to the environmental day-night cycle both behaviourally and physiologically. In animals, not only are complex behaviours such as sleep and mood governed by this oscillator, but also different body functions such as digestion, circulation, and respiration. The basic mechanism of this clock is cell-autonomous in all studied species possessing a circadian clock i.e. each cell contains a network of genes and proteins that can generate an oscillator with a period of approximately 24 hours. These oscillators can, in turn be entrained, to the relevant 24-hour environmental day-night cycle.
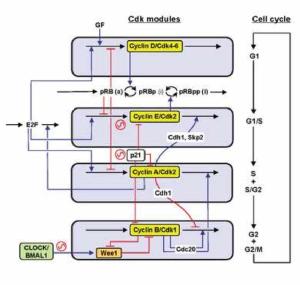
A recent paper Phase-locking and multiple oscillating attractors for the coupled mammalian clock and cell cycle which appeared in PNAS is about the interaction between the mammalian circadian clock and the cell cycle. In the absence of other signals, the cell cycle and circadian clock robustly phase-lock each other in a 1:1 fashion so that in an expanding cell population the two oscillators oscillate in a synchronised way with a common frequency. However, there are additional clock states: as well as the low-period phase-locked state there are distinct coexisting states with a significantly higher period clock and a different frequency ratio.
This underlies one of my current main interests which is to do with the role of the circadian clock in the onset and progression of cancer. My work in this area is with Frances Levi, Robert Dallmann, Barbel Finkenstädt and Annabelle Ballesta.
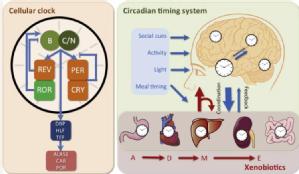
The cell-autonomous circadian clockwork (left) is the functional unit of the CTS, and determines the complex interaction with xenobiotic metabolism. (Left) Simplified core circadian oscillator and one output relevant for xenobiotic metabolism through control of aminolevulinic acid synthase 1 (ALAS1), constitutive androstane receptor (CAR) and cytochrome P450 oxidoreductase (POR). (Right) The CTS involves a central hypothalamic pacemaker – the supra-chiasmatic nuclei (SCN) – which coordinates clocks in all the cells in the body through the generation of an array of physiological rhythms such as rest-activity, body temperature and hormonal secretions. The SCN synchronises the peripheral clocks relative to each other and to the environmental time cues provided by the day-night and social cycles (blue box). Following exposure of an organism to a xenobiotic, the substance undergoes the classical Absorption, Distribution, Metabolism and Elimination (ADME) processes. All of these processes, which ultimately determine the toxicity or pharmacologic effect of the xenobiotic are regulated by peripheral and central clocks present in the gut, heart and blood vessels, liver and pancreas, as well as kidney and colon. Xenobiotics can also reset the molecular clock or CTS through direct interference with the molecular clock or by altering or disrupting physiological pathways.
We are taking a systems approach to chronotherapeutics of cancers, aiming at (i) scaling up and adjusting detailed in vitro chronopharmacology models of the relevant drugs and combinations to the whole organism level, (ii) validating them in mice, and (iii) adjusting them for their implementation in cancer patients. Amongst our aims are the following: (1) To determine the extent of heterogeneity in molecular clocks of cancer cells and circadian coordination in tumour tissues and its impact on (i) cancer progression and (ii) chronotherapy optimization, including combination with clock-targeted pharmacologic or behavioural interventions. (2) To characterise and quantify chronofitness, a novel multidimensional and dynamic CTS biomarker aiming at the prediction of optimal tolerability outcomes on cancer chronotherapy; to maintain/restore/induce chronofitness, through CTS-targeted pharmacologic or behavioural interventions; to determine optimal timing of relevant anticancer drugs in relation to circadian biomarkers within chronofit organisms. (3) To compute and to develop precision chronotherapy delivery algorithms for selected drug combinations in chronofit organisms, through a multiscale systems pharmacology approach integrating heterogeneity in cancer cell clocks, in order to jointly improve anticancer treatment tolerability and efficacy.
