Research
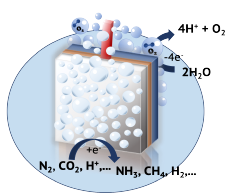 electrocatalyst systems for the photoelectrochemical production of high-valuable chemicals and oxygen. These systems mimic the most important pieces of photosynthesis, nature's go-to solar energy conversion mechanism. Upon photoexcitation, the semiconductor transfers holes or electrons to the integrated electrocatalyst which catalyses the oxidation reaction (e.g., oxygen production) at the (photo-)anode or reduction reaction (hydrogen production, CO2 conversion into long-chain hydrocarbons etc.) at the photocathode. Of particular importance is hereby a careful energetic alignment of the semiconductor-electrocatalyst and the electrocatalyst-electrolyte interface. Furthermore, the electrocatalyst surface composition and topography plays a key role in the catalytic reaction and spectroscopic and optical techniques such as XPS and AFM, HRSEM and HRTEM, resp., are essential tools to investigate catalyst performance and establish routes for its optimization. Current projects are discussed below.
electrocatalyst systems for the photoelectrochemical production of high-valuable chemicals and oxygen. These systems mimic the most important pieces of photosynthesis, nature's go-to solar energy conversion mechanism. Upon photoexcitation, the semiconductor transfers holes or electrons to the integrated electrocatalyst which catalyses the oxidation reaction (e.g., oxygen production) at the (photo-)anode or reduction reaction (hydrogen production, CO2 conversion into long-chain hydrocarbons etc.) at the photocathode. Of particular importance is hereby a careful energetic alignment of the semiconductor-electrocatalyst and the electrocatalyst-electrolyte interface. Furthermore, the electrocatalyst surface composition and topography plays a key role in the catalytic reaction and spectroscopic and optical techniques such as XPS and AFM, HRSEM and HRTEM, resp., are essential tools to investigate catalyst performance and establish routes for its optimization. Current projects are discussed below.Solar fuel and oxygen production in reduced gravitational environment
|
Hydrogen gas bubble evolution on nanostructured p-InP/Rh photocathodes during 9.2s of free fall at the Bremen Drop Tower. |
|

