Recent Publications Feed (ADMIN)
- Insert code for bullet point before paper title - • - and insert a space
- COPY AND PASTE full title into the title field
- In abstract tab insert authors names, year, journal, pages
- In tag box insert authors names separated by a comma
• A practical guide to using boron doped diamond in electrochemical research, Phys. Chem. Chem. Phys., 2015
Conducting, boron doped diamond (BDD), in addition to its superior material properties, offers several notable attributes to the electrochemist making it an intriguing material for electrochemical research. These include the widest solvent window of all electrode materials; low background and capacitive currents; reduced fouling compared to other electrodes and; the ability to withstand extreme potentials, corrosive and high temperature/pressure environments. However, BDD is not your typical electrode material, it is a semi-conductor doped degenerately with boron to present semi-metallic characteristics. Input from materials scientists, chemists and physicists has been required to aid understanding of how to work with this material from an electrochemical viewpoint and improve electrode quality. Importantly, depending on how the BDD has been grown and then subsequently treated, prior to electrochemical measurement, the resulting material properties can vary quite significantly from one electrode to the next. This likely explains the variability seen by different researchers working on the same experimental systems. The aim of this “protocols” article is not to provide a state-of-the-art review of diamond electrochemistry, suitable references are provided to the interested reader, but instead serves as a reference point for any researcher wishing to commence work with diamond electrodes and interpret electrochemical data. It provides information on how best to characterise the material properties of the electrode before use and outlines the interplay between boron dopant density, non-diamond-carbon content, grain morphology, surface chemistry and redox couple identity. All should ideally be considered when interpretating electrochemical data arising from the diamond electrode. This will aid the reader in making meaningful comparisons between data obtained by different researchers using different diamond electrodes. The guide also aims to help educate the researcher in choosing which form of BDD is best suited to their research application.
• Concluding remarks: there's nowt so queer as carbon electrodes, Faraday Discuss., 2014
This contribution provides a personal overview and summary of Faraday Discussion 172 on “Carbon in Electrochemistry”, covering some of the key points made at the meeting within the broader context of other recent developments on carbon materials for electrochemical applications. Although carbon electrodes have a long history of use in electrochemistry, methods and techniques are only just becoming available that can test long-established models and identify key features for further exploration. This Discussion has highlighted the need for a better understanding of the impact of surface structure, defects, local density of electronic states, and surface functionality and contamination, in order to advance fundamental knowledge of various electrochemical processes and phenomena at carbon electrodes. These developments cut across important materials such as graphene, carbon nanotubes, conducting diamond and high surface area carbon materials. With more detailed pictures of structural and electronic controls of electrochemistry at carbon electrodes (and electrodes generally), will come rational advances in various technological applications, from sensors to energy technology (particularly batteries, supercapacitors and fuel cells), that have been well-illustrated at this Discussion
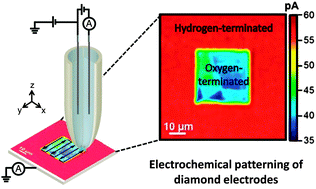
• Electrochemical "read-write" microscale patterning of boron doped diamond electrodes, Chem. Comm., 2014
Scanning electrochemical cell microscopy is utilised as a read–write pipette-based probe to both electrochemically modify the local surface chemistry of boron doped diamond and “read” the resulting modification, at the micron scale. In this specific application, localised electrochemical oxidation results in conversion of the H-terminated surface to –O, electrochemically visualised by monitoring the current change for reduction of Ru(NH3)63+. This methodology, in general, provides a platform for read–write analysis of electrodes, opening up new analytical avenues, particularly as the pipette can be viewed as a microfluidic device.