Recent Publications Feed (ADMIN)
- Insert code for bullet point before paper title - • - and insert a space
- COPY AND PASTE full title into the title field
- In abstract tab insert authors names, year, journal, pages
- In tag box insert authors names separated by a comma
• Nucleation, aggregative growth and detachment of metal nanoparticles during electrodeposition at electrode surfaces, Chemical Science, 2014
The nucleation and growth of metal nanoparticles (NPs) on surfaces is of considerable interest with regard to creating functional interfaces with myriad applications. Yet, key features of these processes remain elusive and are undergoing revision. Here, the mechanism of the electrodeposition of silver on basal plane highly oriented pyrolytic graphite (HOPG) is investigated as a model system on a wide range of length scales, spanning electrochemical measurements from the macroscale to the nanoscale using scanning electrochemical cell microscopy (SECCM), a pipette-based approach. The macroscale measurements show that the nucleation process cannot be modelled as either truly instantaneous or progressive, and that step edge sites of HOPG do not play a dominant role in nucleation events compared to the HOPG basal plane, as has been widely proposed. Moreover, nucleation numbers extracted from electrochemical analysis do not match those determined by atomic force microscopy (AFM). The high time and spatial resolution of the nanoscale pipette set-up reveals individual nucleation and growth events at the graphite basal surface that are resolved and analysed in detail. Based on these results, corroborated with complementary microscopy measurements, we propose that a nucleation-aggregative growth-detachment mechanism is an important feature of the electrodeposition of silver NPs on HOPG. These findings have major implications for NP electrodeposition and for understanding electrochemical processes at graphitic materials generally.
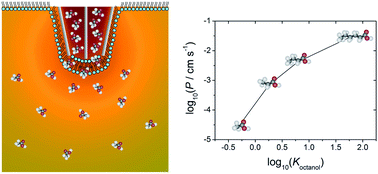
• A new approach for the fabrication of microscale lipid bilayers at glass pipets: application to quantitative passive permeation visualization, Soft Matter, 2014
A new method of planar bilayer lipid membrane (BLM) formation is presented that allows stable, solvent-free lipid bilayers exhibiting high seal resistances to be formed rapidly, easily and reproducibly. Using these bilayers the passive permeation of a series of carboxylic acids is investigated, to determine quantitatively the trend in permeability with lipophilicity of the acid. BLMs are formed at the tip openings of pulled theta pipets, and the rate of permeation of each carboxylic acid across the bilayer, from within the pipet into the bulk solution is determined. This is achieved through spatially-resolved measurements of the pH change that occurs upon the permeation of the weak acid, visualized using a pH-sensitive fluorophore with a confocal laser scanning microscope. The extracted fluorescence profiles are matched to finite element method (FEM) simulations, to allow the associated permeation coefficient for each weak acid to be determined with high accuracy, since this is the only adjustable parameter used to fit the experimental data. For bilayers formed in this way, the weak acids show increasing permeability with lipophilicity. Furthermore, the arrangement allows the effect of a trans-membrane electric field on permeation to be explored. For both propanoic and hexanoic acid it is found that an applied electric field enhances molecular transport, which is attributed to the formation of pores within the membrane.
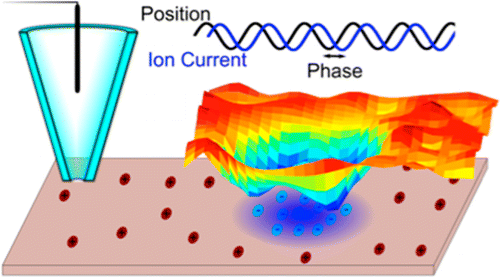
• Surface Charge Mapping with a Nanopipette, J. Am. Chem. Soc., 2014
Nanopipettes are emerging as simple but powerful tools for probing chemistry at the nanoscale. In this contribution the use of nanopipettes for simultaneous surface charge mapping and topographical imaging is demonstrated, using a scanning ion conductance microscopy (SICM) format. When a nanopipette is positioned close to a surface in electrolyte solution, the direct ion current (DC), driven by an applied bias between a quasi-reference counter electrode (QRCE) in the nanopipette and a second QRCE in the bulk solution, is sensitive to surface charge. The charge sensitivity arises because the diffuse double layers at the nanopipette and the surface interact, creating a perm-selective region which becomes increasingly significant at low ionic strengths (10 mM 1:1 aqueous electrolyte herein). This leads to a polarity-dependent ion current and surface-induced rectification as the bias is varied. Using distance-modulated SICM, which induces an alternating ion current component (AC) by periodically modulating the distance between the nanopipette and the surface, the effect of surface charge on the DC and AC is explored and rationalized. The impact of surface charge on the AC phase (with respect to the driving sinusoidal signal) is highlighted in particular; this quantity shows a shift that is highly sensitive to interfacial charge and provides the basis for visualizing charge simultaneously with topography. The studies herein highlight the use of nanopipettes for functional imaging with applications from cell biology to materials characterization where understanding surface charge is of key importance. They also provide a framework for the design of SICM experiments, which may be convoluted by topographical and surface charge effects, especially for small nanopipettes.