MChem Research
The following work comprised 50% of the fourth and final year of my undergraduate MChem degree in Chemical Biology.
Introduction
Title: Gluco-functional Polymers/Particles and Study of their Interactions with the GLUT-1 Transporter
Cancer is a leading cause of premature death in humans, requiring therapies that target “hallmark” cancer cell traits to specifically impede cancer cells without damaging “normal” cells. Tumour cells exhibit an increased demand for nutrients, overexpressing the GLUT-1 glucose transporter to enable increased glucose entry. Glycoconjugates (drugs with sugars that can interact with the transporter attached) can exploit this overexpression to enhance specific targeting.
Experimental
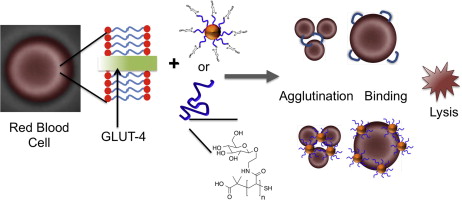
This study focuses on glucose-conjugated polymers and gold nanoparticles (AuNPs) conjugated at the tolerated 1- and 2-position of glucose. Erythrocytes (red blood cells) exclusively express the GLUT-1 transporter, and therefore can be used to assess GLUT-1 interaction. Protected glycosylated N-Hydroxyethyl acrylamide (HEA) monomer (Glu) was synthesized, using a boron trifluoride catalyst, polymerised and deprotected to produce glycopolymers of various chain lengths that were then labelled with FITC through physisorption.
Non-glycosylated HEA polymers of various chain lengths were conjugated to AuNPs and glucose functionality was attached to their chain ends by coupling reactions (EDC/NHS coupling). These latter polymers and particles were used as controls to assess specific interaction with the sugar moieties.
Results
Cytotoxicity assays suggested that sheep erythrocytes were tolerant of the glycopolymers. Aggregation and morphology assays undertaken by bright field microscopy showed no aggregation with any of the glucose-functional polymers or AuNPs, and morphology changes and lysed “ghost” cell populations were not significantly greater than in phosphate-buffered saline (PBS) negative controls. Polymer chain length did not have a noticeable effect.
Fluorescence microscopy was used to analyse the interaction of the fluorescein isothiocyanate-labelled polymers. No greater FITC fluorescence was present in erythrocytes when attached to PGlu polymer relative to FITC controls, indicating that the FITC labelling of the erythrocytes may be due purely to non-specific FITC labelling. Ghost cells appeared to be specifically targeted by the labelled PGlus, suggesting some specific interaction with the cell surface transporter causing haemolysis, but this is unclear. Covalently-attached fluorescent labels and more stringent purification are required to assess interaction fully.
This lack of significant interaction complies with previous studies, suggesting that the synthesized glycoconjugates are biocompatible with erythrocytes. Their lack of undesirable interactions highlights their potential for delivery and imaging applications.
