Gibson Group News
Welcome to new group member, Qiao
Qiao Tang has joined the group as a PhD student in January, on a prestigious Chancellors International Scholarship. Qiao's research will explore the biomedical application, glycan metabolic labelling and its use to capture synthetic nanomaterials and polymers.
Matt awarded the 2021 McBain Medal!
Matt has been awarded the 2021 McBain Medal from the Society for Chemical Industry and Royal Society of Chemistry. This award is to "honour an early career researcher or technologist who has made a meritorious contribution to colloid and interface science." Matt was particularly pleased that he can still be called Early Career. There were will be a special symposia late in 2021 where Matt will receive the medal and give a lecture.
This medal represents the massive contributions of past and current team members which are too numerous to list. Thanks go to UoW and both Department of Chemistry and Medical School for allowing the GibsonGroup to spread their work over both Departments.
Fluoro-glycans to improve glyconanoparticle selectivity is published
Glycans (aka sugars, carbohydrates) direct many recognition and signalling processes in biology. Multivalency (presentation of lots of copies) is crucial to overcome glycans intrinsic low affinity, hence materials (polymers, particles, surfaces) which display them are appealing probes of function, or as new diagnostics (e.g. see our work on COVID diagnostics). However, most studies use simple monosaccharides, which may not have selectivity or are only tested against plant proteins. In this work, we collaborated with teams from Bristol, York and Southampton - our collaborators developed a chemoenzymatic synthesis to obtained selectively fluorinated glycans based on lacto-N-biose. Fluorine is appealing as it is small, does no have significant effects on conformation, but can change hydrogen bonding patterns. These glycans were incorporated into our polymer-stabilised nanoparticle platform, and found to modulate the affinity towards 2 galectins -an important class of galactose-binding biomarkers. This work shows that unnatural glycan-functional nanoparticles could be deployed as biosensors.
Read the paper here;
Controlling Dendritic Cell Function using Glyco-surfaces is published
There is a real need to modulate our immune systems to help treat cancer, autoimmune disorders and allergies. One of the key cell types in immune responses are dendritic cells. There is particular interest in how dendritic cells interact with, and respond to glycans (sugars), which is a key process during e.g pathogen recognition. In this work we developed surfaces bearing different monosaccharides, attached via a polymeric tether and our collaborators at Nottingham University investigated the impact this had on dendritic cell function. The strategy was crucial as no soluble additives were used, so the signalling was purely from the cell/solid interface, and hence would show if a material can be used to tune DC cell function. The key results were that specific combination of glycans could suppress dendritic cell activation implying an anti-inflammatory or regulatory phenotype.
Read the paper here
Polymer/protein conjugates to engineer freeze stability is published
Proteins find application as catalysts, therapies, in food stuff and in diagnostics. However, they often require cold storage, with organic solvents used as excipients to protect them. In our latest work, we further explore the use of PVA (poly(vinyl alcohol) as an additive to control ice growth (ice recrystallisation inhibition) and hence protect proteins. Here we first explore how PVA can protect LDH (lactate dehydrogenase) which is a hard-to-freeze enzyme. We then explore whether simply mixing, or covalent attaching, the PVA to a protein gives maximal protection. Bioconjugation with PVA is hard, as it is a lesser activated monomer and it must be polymerized as vinyl acetate - the deprotection step complicates the synthetic design. Therefore we used non-site specific methods (targeting amines) to attach the PVA, in what is only the second ever report (we think) of a PVA-protein conjugate. The conjugation of the polymer provided protection at lower concentrations than simple mixing and shows that PVA might be an appealing alternative to e.g. PEGylation, as it brings advanced cryoprotectant properties.
Read the paper here
Welcome to new group member, Dr Marta Neves
Marta joined the University of Warwick and GibsonGroup as an Institute of Advanced Study WIRL-COFUND Fellow in September 2020. Marta was born in Portugal and she has a PhD in Pharmaceutical Sciences from the University of Porto (Portugal). Marta has a background in electrochemical biosensing which she acquired working across international and inter-sectorial environments. At GibsonGroup, Marta is going to study prostate cancer glycobiology and design and develop novel multiplexed biosensors to overcome limitations of current diagnostic approaches
SARS-COV-2 detection paper now published in ACS Central Science
There is an urgent, global, need for new therapeutic, vaccine and diagnostic interventions to address the COVID-19 challenge. Current diagnostics are mostly based upon PCR (polymerase chain reaction) methods where the genetic material of the SARS-COV-2 virus is isolated and sequenced. A challenge with this method is that significant infrastructure and trained personnel are needed, and the results are not instant. In this work, conducted in collaboration with Iceni Diagnostics (and MANY UoW colleagues) we hijacked a pregnancy test set up, to enable rapid detection. Crucial to this was the identification that sialic acids (a type of cell-surface glycan) bind the SARS-COV_2 spike protein. By incorporating sialic acids onto the ends of polymers, immobilized onto gold nanoparticles we made a paper-based tool, enabling rapid detection of the spike protein, a virus mimic and also a virus engineered to 'look like' SARS-COV-2. This method may enable ultra rapid and low cost screening to identify individuals who carry the virus, to triage for the PCR testing. We are actively pursuing the development of this technology.
Read the paper here
Synthesis of glyconanoparticles without protecting groups, published in Bioconjugate Chemistry
Glycans (sugars) dictate a huge range of biological processes, from host-pathogen interactions to cell-cell communication. We have a large research 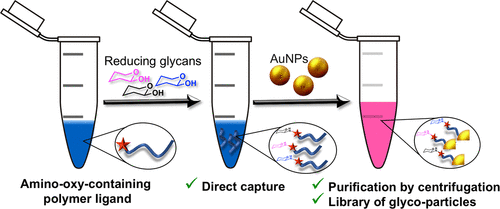
program into using nanomaterials to display glycans, mimicking how they are presented on cell surfaces. For example, we have used these in diagnostics (including our recent work on rapid COVID diagnostics). However a key challenge is actually getting the glycans on the nanoparticles as multiple protecting groups are often needed. In our latest paper, we installed amino-oxy groups at the end-group of polymers to allow capture of reducing glycans in aqueous solution, with the aim of simplifying our synthetic route with our long-term aim of fully-automating the process. We critically evaluated the efficiency of this reaction using 13-C enriched glucose, to enable the actual polymer, rather than model small molecules to be used. This show 25 % of the chains can capture a glycan, which was then immobilised onto gold nanoparticles. This approach is particularly suited to screening applications where small amounts of glycan are available, to identify hits.
Read the paper here
Protecting Group Free Synthesis of Glyconanoparticles Using Aminooxy-Terminated Polymer Ligands
