Trial documentation
On this page you will find all relevant documents and resources for setting up and conducting the trial at site.
To help you with this, please find a version control log listing all of our documents here. If you wish to print these documents to create a paper ISF, please find an index here.
Thank you to all of our sites for your hard work so far. Please find a certificate of appreciation here.
Protocol
Participants Documents
Main Ethics
Amendments
Substantial Amendments
Non-Substantial Amendments
Documents for Site Greenlight
Data Collection
Participant Safety
Useful Documents/Guides
Working Instructions
Protocol
Participants Documents
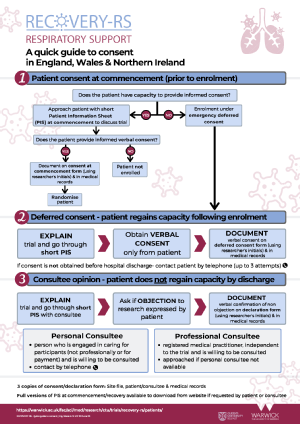
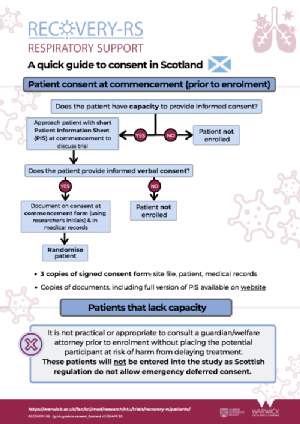
Consenting patient prior to treatment
Consent form (on commencement)
Patient Information Leaflet (on commencement)
Short Patient Information Leaflet (on commencement)
Easy Read PIS (print)
Easy Read PIS (screen)
Deferred consent
Patient Information Sheet
Short Patient Information Leaflet
Consultee Declaration Form & Cover Letter
Deferred Consent Form
Translated Documents
Main Ethics
IRAS Organisation Information Document
Amendments
Substantial Amendments
-
Substantial amendment #1
IRAS Notice of Substantial Amendment_SA#01_21 April 2020
REC approval letter_SA#-01_21 April 2020
HRA Approval Letter_SA#01_21 April 2020
Summary of Changes_SA#01_21 April 2020
-
- Consent process updated to enable consent prior to enrolment where patient has capacity
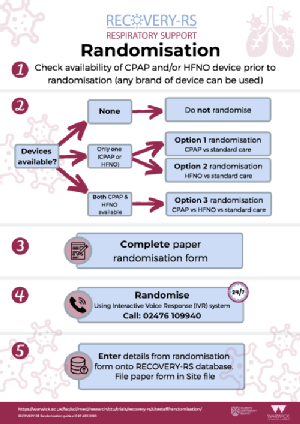
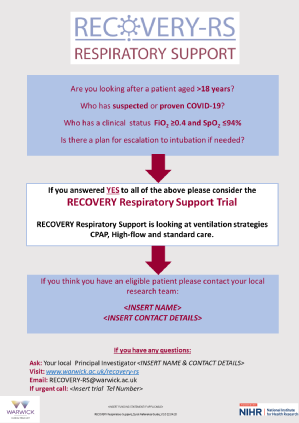
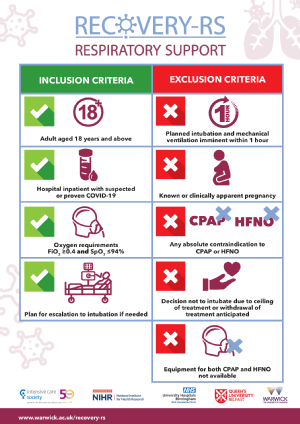
- Eligibility criteria update to clarify that patients will be eligible with FiO2 more than or equal to 0.4 and SpO2 less than or equal to 94%.
- Update to protocol to include process for Scottish sites
- All Patient documentation updated to include reference to collection of race and ethnicity data
- New information sheets and consent forms added for obtaining verbal informed consent from patients with capacity, prior to randomisation
- All changes are referenced in full in summary of changes document
-
Substantial amendment #2
SA02 applies only to certain sites.
IRAS Notice of Substantial Amendment_SA02_15 May 2020
HRA Approval Letter_SA02_15 May 2020
-
Substantial amendment #3
IRAS Notice of Substantial Amendment_SA#03_01 June 2020
REC approval letter_SA#03_01 June 2020
HRA Approval SA#03_01 June 2020
Summary of Changes_SA#03_June 2020
-
- Amendment to Protocol, from v3.1 to v4.0
- Addition of an Easy Read PIS (screen & print)
- Minor updates to Consultee Consent Form, Consultee Cover Letter, PIS, PIS on Commencement Short PIS & Short PIS on Commencement
Non-Substantial Amendments
-
Non-substantial amendment #1
Sponsor Approval Letter - NSA 01
8th April 2020. Addition of sites. Minor amendment to deferred consent form.
-
Non-substantial amendment #2
Sponsor Approval Letter - NSA 02
14th April 2020. Addition of sites.
-
Non-substantial amendment #3
Sponsor Approval Letter - NSA 03
15th April 2020. Addition of sites.
-
Non-substantial amendment #4
Sponsor Approval Letter - NSA 04
16th April 2020. Addition of sites.
-
Non-substantial amendment #5
Sponsor Approval Letter - NSA 05
17th April 2020. Addition of sites. Minor amendments to PILs and Assent form. Correction of typographical error in Assent form. Minor amendments to consent forms.
-
Non-substantial amendment #6
Sponsor Approval Letter - NSA 06
21st April 2020. Addition of sites.
-
Non-substantial amendment #7
Sponsor Approval Letter - NSA 07
24th April 2020. Addition of sites.
-
Non-substantial amendment #8
Sponsor Approval Letter - NSA 08
24th April 2020. Addition of sites. Minor amendments to Protocol. Minor amendments to PILs.
-
Non-substantial amendment #9
Sponsor Approval Letter - NSA 09
28th April 2020. Addition of sites.
-
Non-substantial amendment #10
Sponsor Approval Letter - NSA 10
30th April 2020. Addition of sites. Addition of twitter account.
-
Non-substantial amendment #11
Sponsor Approval Letter - NSA 11
4th May 2020. Addition of sites.
-
Non-substantial amendment #12
Sponsor Approval Letter - NSA 12
6th May 2020. Addition of sites.
-
Non-substantial amendment #13
Sponsor Approval Letter - NSA 13
11th June 2020. Minor amendment to Consultee Consent Form. Addition of translated PILs and Consent Forms in French, Portuguese, Polish & Urdu.
-
Non-substantial amendment #14
Sponsor Approval Letter - NSA 14
2nd July 2020. Addition of sites.
-
Non-substantial amendment #15
Sponsor Approval Letter - NSA 15
2nd July 2020. Addition of sites.
-
Non-substantial amendment #16
Sponsor Approval Letter - NSA 16
20th August 2020. Translated documents.
-
Non-substantial amendment #17
Sponsor Approval Document - NSA 17
3rd November 2020. Addition of site. New PI at site.
-
Non-substantial amendment #18
Sponsor Approval Document - NSA 18
23rd November 2020. New PIs at site.
-
Non-substantial amendment #19
Sponsor Approval Document - NSA 19
21st September 2021. New PIs at site.
Documents for Site Greenlight
back to top
Data Collection
back to top
Participant Safety
back to top
Useful Documents/Guides
|
|
Infographic: Trial Flexibility
|
back to top
Working Instructions
SAE Handling Process Working Instruction