RECOVERY-RS Respiratory Support : Respiratory Strategies in COVID-19; CPAP, High-flow, and standard care
THIS URGENT PUBLIC HEALTH NATIONAL CLINICAL TRIAL AIMS TO IDENTIFY TREATMENTS THAT MAY BE BENEFICIAL FOR ADULTS HOSPITALISED WITH SUSPECTED OR CONFIRMED COVID-19.
A range of potential treatments have been suggested for COVID-19 but nobody knows if any of them will turn out to be more effective in helping people recover than the usual standard of hospital care which all patients will receive. The RECOVERY-RS trial will compare the effectiveness of three ventilation methods;
- Continuous positive airway pressure (CPAP): this treatment applies mild to high air pressure on a continuous basis through a tightly fitted face mask. It keeps the airways continuously open in people who are able to breathe normally on their own, but need help keeping their airway clear.
- High flow nasal oxygen (HFNO): this is a way of giving humidified (moistened) and warmed oxygen through tubes into the nose. The oxygen is delivered very quickly to help patients who have low oxygen levels and find breathing on their own difficult.
- Standard care: standard treatment will involve oxygen delivered via a normal face mask or tubes in the nose.
Data from the trial will be regularly reviewed so that any effective treatment can be identified quickly and made available to all patients.
Latest news - click here!
09/02/2021
RECOVERY-RS is now the largest global non-invasive respiratory support trial for COVID-19 with 1,106 patients recruited as of this morning. We would like to say a huge thank you to all the staff, patients and families involved for helping us reach this incredible milestone!
01/02/2021
The DMC for RECOVERY-RS met at the end of January. They noted the considerable progress that had been made since the last DMC meeting, with over 1000 participants now enrolled, making the trial the world’s largest trial of non-invasive ventilation in acute respiratory failure. After careful consideration of the available data, the committee recommended that recruitment continues. A further review will be considered in 4 weeks time. The committee requested that we prioritise obtaining the outcome data for those enrolled to date. We plan to work with NHS Digital, ICNARC and our recruiting sites to obtain as much information as possible to inform the next review.
The investigator team would like to extend their sincere thanks to the patients, families, clinicians and research teams who have supported the trial to date. We plan to provide a further update following the next interim analysis.
RECOVERY-RS Team.
Why is this trial needed?
Support for RECOVERY-RS
Letter from the Chief Medical Officers
The Chief Medical Officers of England, Wales, Scotland and Northern Ireland, and the
NHS Medical Director, have written a letter to all doctors strongly encouraging participation
in the national randomised trials in COVID-19 of which RECOVERY-RS is one. Please click on the link below to read the letter and pass it on to your colleagues:
The importance of COVID-19 clinical trials.
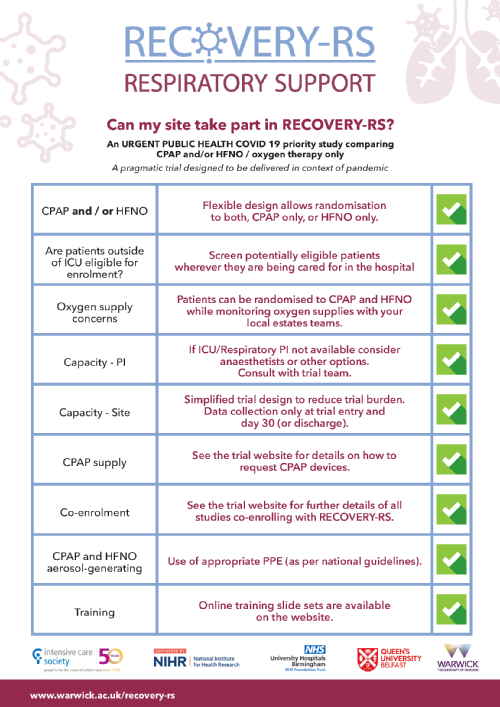
Can my hospital site take part in RECOVERY-RS?

Trial News
Information for Patients
Information Sheets and consent forms
FAQs
Information for Site Staff
Confirmation of online training form
Publications
Chief Investigator:
Prof Gavin Perkins
Co-Chief Investigator:
Prof Danny McAuley
Sponsor:
Funder:
Registration Number:
Enquiries:
Please direct all enquiries to the RECOVERY-RS team;
Email: RECOVERY-RS@warwick.ac.uk