Alistair Irvine
Before MOAC
My undergraduate degree was in Computing Science at the Magee campus of the University of Ulster in Londonderry. This included a sandwich year with British Telecommunications in Belfast. After working in Belfast for another IT company, Liberty IT, I decided I'd had enough and left to travel for a year. This included working for 3 months with Raleigh International in the Patagonia region of Chile. I spent another couple of months roaming around Argentina and Peru, before spending 5 month working and travelling around Australia.
When I finally arrived back in the UK, I spent the next year doing an MSc in Bioinformatics at the University of Manchester. This included a couple of months on a research project at the International Livestock Research Institute (ILRI) in Nairobi, Kenya.
MSc
There's a good atmosphere at MOAC and I'm enjoying studying here. My first year here was very rewarding. During the MSc I spend two months on each of the follwing research projects, each giving experience into a different aspect of the multi-disipline approach to the life sciences:
Mathematics/Computing: Signal peptide prediction in archaea using profile hidden Markov models.
Biophysical Chemistry: Pruification and characterisation of transmembrane domains.
Experimental Biology: The Pho regulon of Myxococcus xanthus.
PhD Project
Title: Interaction of a folding enzyme with a partly-folded protein substrate
Supervisors: Prof. Robert Freedman (Biological Sciences) and Dr. Claudia Blindauer (Chemistry)
(Biological Sciences) and Dr. Claudia Blindauer (Chemistry)
Advisors: Ann Dixon (Chemistry); Corrine Smith (Biological Sciences); Dave Roper (Biological Sciences)
Aim: To understand the structures and properties of protein folding intermediates and how the process of folding is facilitated by molecular catalysts
Technologies Used: Solution state NMR, mass spectrometry
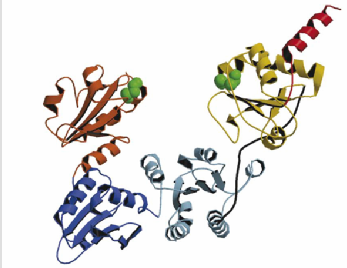
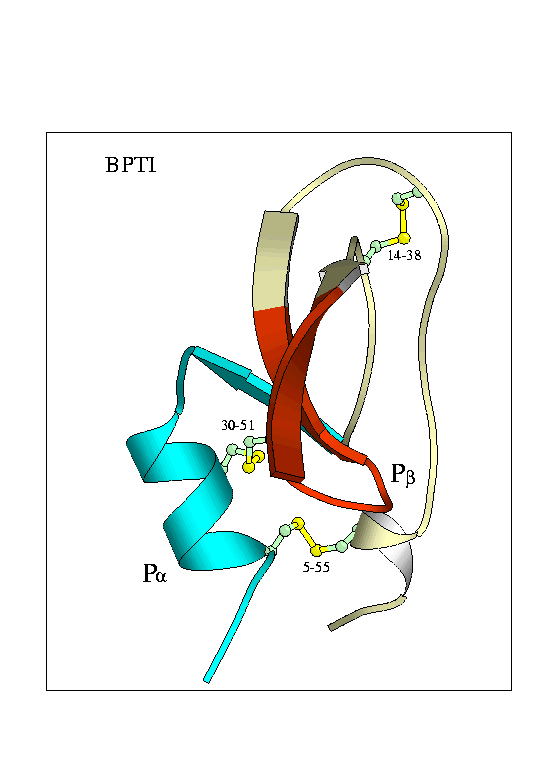
The proteins I'm studying are the folding enzyme Protein Disulphide Isomerase (PDI) and a substrate protein Bovine Pancreatic Trypsin Inhibitor (BPTI)
 |
|
Tertiary Structure of Bovine Pancreatic Trypsin Inhibitor (BPTI) and Protein Disulphide Isomerase
Summary
Fundamental to the structure of many proteins is the formation of disulphide bridges - covalent bonds between the sulphur atoms of cysteine residues. An enzyme known as Protein Disulphide Isomerase (PDI) catalyses the formation of disulphide bonds. It also helps in exchange of disulphide bonds from one pair of cysteines to another - a process essential in the folding pathway of many proteins. This study investigates the folding pathway of Bovine Pancreatic Trypsin Inhibitor (BPTI), a small protein containing 3 native disulphide bonds. Particular focus will be on the interaction of the BPTI protein with the PDI enzyme. By creating a variety of cys-removed mutant forms of the protein we can capture the enzyme-protein interaction at various stages along the folding pathway.
Other Interests
Convservation work: British Trust for Conservation Volunteers (BTCV)
Jogging: Coventry and Birmingham half marathons.
Sports: football, squash, badminton

MSc Graduation

Bowling with friends

My nephews & niece

Conservation Work