Mini-Project 3
Probing Bacterial-Toxin Inhibition with Synthetic Glycopolymers Prepared by Tandem Post-Polymerization Modification: Role of Linker Length and Carbohydrate Density
Supervised by: Dr Matthew Gibson
This work was published: Richards, S-J., Jones, MW, Hunaban, M., Haddleton, DM., Gibson, M.I., Angewandte Chemie. International Edition., 2012, 51, 7812 - 7816
The cholera toxin (CTx) secreted by Vibrio cholerae binds glycosides expressed on the cell surface. Materials with high-affinity and selectivity for these lectins could find applications as anti-adhesive agents.
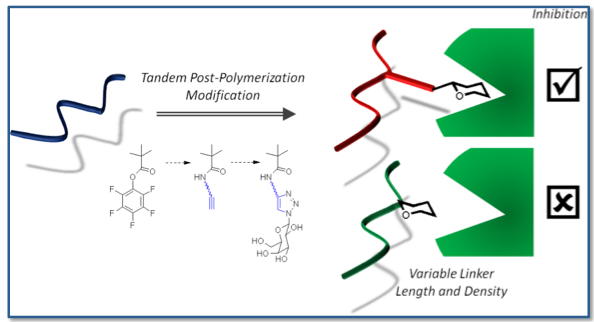
Probe influence of chain length, carbohydrate density and linker length on binding inhibition.
Glycopolymer library produced by tandem post - polymerisation modifications.
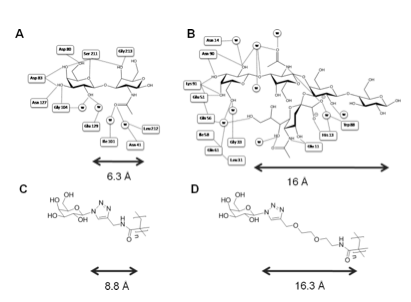
Structural biology indicates CTx has deeper binding site than other galactose binding lectins such as Peanut agglutinin (PNA).
‘Clickable’ units are not compatible with controlled radical polymerisation. Instead, tandem-post polymerisation modification techniques were used.
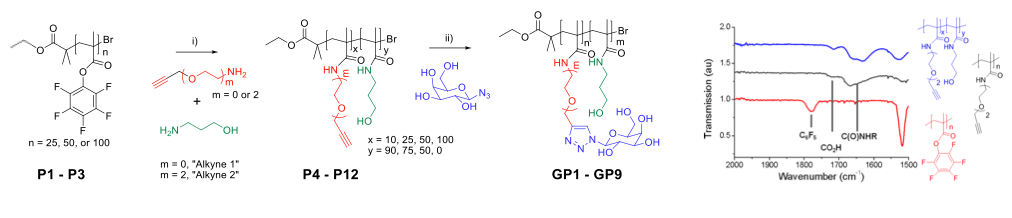
Poly(pentafluorophenyl methacrylate) for easy modification.
β-D-galactose was ‘clicked-on’ to pendent alkyne moieties.
Results in biocompatible methacrylamide based (co)polymers.
Polymers synthesised with ~ 6 Å (short) and 16 Å (long) linkers.
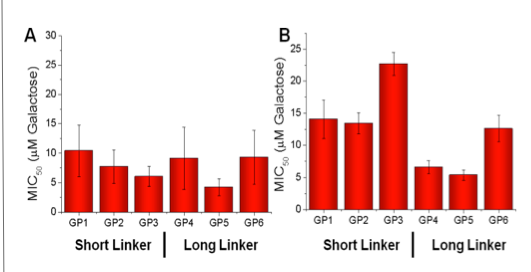
Linker length has no effect on PNA inhibition (A).
Longer linker has 2 – 3 fold lower MIC50 compared to shorter linker on inhibiting CTx (B).
100 X more active than free galactose.
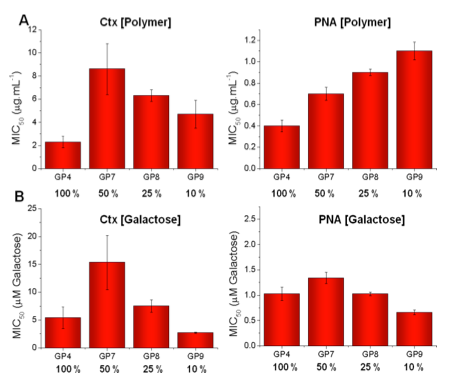
Polymers synthesised with 10, 25 and 50 % galactose.
By mass (A), low galactose densities lead to a relative decrease in binding affinity/inhibitory activity.
By mole of galactose (B), 10 % and 100 % functionalised polymers are the most active suggesting several features (e.g. sterics and site spanning) contribute to inhibitory activity.
