Ph.D Research
Exploiting natural and semi-synthetic toxin delivery systems for drug transport
The aim of my PhD project is to investigate the potential for functionalisation of a novel protein nano-scale syringe produced endogenously by the Photorhabdus species of bacteria (with homologues among other Enterobacteriaceae) - the "Photorhabdus Virulence Cassette (PVC)". Additionally, we are exploring the possibility of engineering other toxin systems such as binary A-B toxins (for instance cholera toxin (CTX) to the same end.
Functionalisation of structures such as these are very sparely reported in the literature, and all currently existing systems that attempt to utilise bacterial secretion systems have the fatal flaw of requiring live bacteria. Moreover, while exploitation of AB toxins is more heavily researched, to our knowledge, no one is attempting to use them for delivery of bioactive drugs and molecules of interest - research so far has largely focussed on conjugation to nanoparticles or imaging aids.
Broadly speaking we would like to:
- Use synthetic biology and bio-orthogonal chemistry approaches to confer bespoke functionality to the structures.
- Obtain further structural and mechanistic information (e.g. via high-resolution imaging).
- Alter target specificity in order to apply the structures to any given cell type.
- Alter payload molecules (e.g. apply other toxins or growth factors for cytotoxicity or stem cell differentiation).
Photorhabdus in popular media:
 A New Scientist article was published in 2012 which discusses the nature and applications of the PVCs in simple terms (image credit)[1]:
A New Scientist article was published in 2012 which discusses the nature and applications of the PVCs in simple terms (image credit)[1]:
Photorhabdus also topped the list of BBC Earth's 'bacteria with super powers':
Mental_Floss covered an interesting and little known aspect of Photorhabdus: the so called "Angel's Glow"
A bit of Background on our Bug
Photorhabdus are a genus of insect pathogens and nematode worm symbionts[2], with some species within the clade exhibiting opportunistic human pathogenesis (see the figures below)[3]. The genus is gaining traction as a model organism for the study of host - pathogen interactions owing to its diverse and adaptable lifestyle. Another particularly interesting facet of Photorhabdus biology is the broad range of toxins and antimicrobial secondary metabolites they produce. In fact, previous studies have shown that a greater proportion of the Photorhabdus genome is given over to production of these compounds than the current model organisms, Streptomyces spp. Recently, a novel structure was identified in this genus involved in the secretion and delivery of toxin molecules. A homologous structure of bacteriophage tails and the Type 6 Secretion System, it has since been identified in a number of Enterobacteriaceae. Dubbed the Photorhabdus Virulence Cassette (PVC), a number exist in each species within the genus, and is comprised of 15 structural genes and a 'payload' toxin gene. When expressed and assembled, they form a nano-scale, toxin-loaded, protein syringe which is secreted by the cell and provides specificity and extremely specific doses. Unsurprisingly, these may make for extremely useful and advanced drug delivery systems if they can be sucessfully reverse engineered.
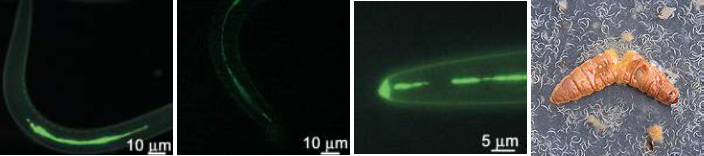
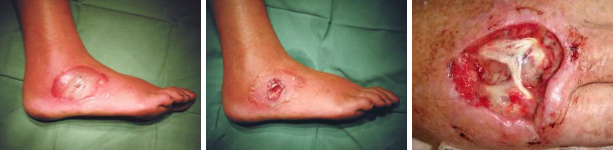
The above images show some of the life cycle of the bacterium, in its nematode host (top)[4], emerging from a wax moth larva (top right) and the ulcers caused during human infection (bottom row)[3,4] that earn it it's status as a symbiont and pathogen of multiple species.
The Photorhabdus Virulence Cassette
Richard Feynman once famously delivered, in his 1959 lectures at Caltech, a seminar entitled "There's plenty of room at the bottom". He was referring mainly to the possibility of us building machines that could manipulate individual atoms. However, he also discusses chemical engineering by physical manipulation on a nano-scale - 'true' nanomachines. Fortunately, Nature does this routinely - in the form of proteins and protein complexes[6]. The particular structure of interest in this project can be seen in the schematic diagram adjacent[3]. A complex of various proteins with differing roles, we are hoping to utilise this nano-scale 'needle' for advanced drug delivery techniques - nano-scale chemical manipulation.
The PVC is the only known secretion system that is, itself, secreted. One barrier to the usage of bacterial secretion systems for therapeutics would be the need for live bacteria to be present. This carries risk, and if they were to be used therapeutically, you'd have a hard time convincing people that being dosed with bacteria will help them! Consequently, the PVCs are an ideal target to explore for advanced drug delivery methods.
Below is the first poster made for this project, imitating a Haynes' Manual and their exploded diagrams, the constituent parts of the PVC are shown. THe poster won a prize at the 2015 MOAC Annual Conference.
References and Further Reading Materials
[1] Roberta Kwok. Driller Killers. The New Scientist, 2012.
[2] N R Waterfield, B W Wren, and R H Ffrench-Constant. Invertebrates as a source of emerging human pathogens. Nature reviews: Microbiology, 2(10):833–41, 2004.
[3] N R Waterfield, T A Ciche, and D J Clarke. Photorhabdus and a host of hosts. Annual Review of Microbiology, 63:557–74, 2009.
[4] A S Weissfeld, R J Halliday, D E Simmons, E A Trevino, P H Vance, C M O Hara, E G Sowers, R Kern, R D Koy, M Bing, C Lo, J Gerrard, R Vohra, J Harper, and K Hodde. Photorhabdus asymbiotica , a pathogen emerging on two continents that proves that there is no substitute for a well-trained clinical microbiologist. Journal of Clinical Microbiology, 43(4152-4155):1–5, 2005.
[5] T A Ciche and J C Ensign. For the Insect Pathogen Photorhabdus luminescens, Which End of a Nematode Is Out? Applied and Environmental Microbiology, 69(4):1890–1897, 2003.
[6] E Gazit. Plenty of room for biology at the bottom: An introduction to bionanotechnology, 1st Edition. Imperial College Press. London, 2007.
