Ultrafast Photochemistry of Sunscreens
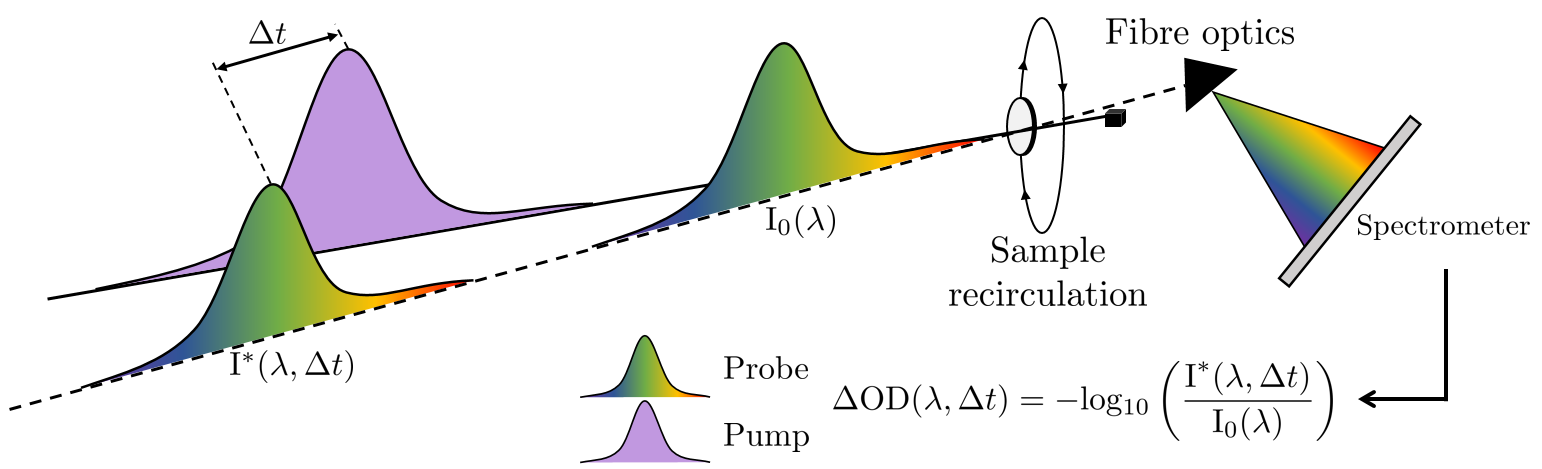
The technique
We observe the effect of light-matter interactions in a range of UV chromophores which are often found in commercial sunscreens, using UV-visible transient electronic absorption spectroscopy (TEAS).
Briefly, we use two femtosecond lasers, one to prepare molecule in their excited state, referred to as the pump pulse, and another to probe the excited state molecules, called the probe pulse. The probe pulse is a broadband white light continuum and is sent in after a time delay () relative to the pump pulse, and consequently, we observe the dynamics of the excited state molecule at a time
after initial photoexcitation. Varying the time delay, typically from fs <
< ns allows us to observe the dissipation mechanism these molecules display in this time window. We measure the change in optical density (OD), that is, the intensity of the probe pulse before and after photoexcitation by the pump pulse.
Sunscreens
The class of molecules we are focusing on are UV chromophores. Specifically, we are looking at such molecules which are used in many commercial sunscreen products. Sunscreens are used to act preemptively against an over exposure to UV radiation, which is known, for instance, to be responsible for the development of malignant melanomas. Applied sunscreens work in addition to the body’s delayed natural photoprotective mechanism: tanning, which utilises a network of melanocytes which synthesise and disperse melanin to surrounding cells.
Often with these molecules, they are selected for their strong absorption cross section in the UV region, but typically, the mechanism by which they dissipate their energy after UV excitation remains some what unclear. This is where these experiments come in. We aim to understand how energy is dissipated in these molecules, which is the first step in understanding not only why these molecules are good at their job, but it also helps us to gauge the safety and thus suitability these molecules are as a sunscreen constituent.

The molecules
Oxybenzone
Oxybenzone is an active ingredient present in many commercially available sunscreens. It displays broadband UV absorption (spanning the UVA and UVB regions) and remains photostable after several hours of irradiation. We used TEAS, as described above, along with a complementary technique, transient vibrational absorption spectroscopy (TVAS) in collaboration with the Ashfold group in Bristol and guided by ab initio electronic structure calculations we shed light on the energy dissipation mechanism oxybenzone displays after UVA irradiation [1].
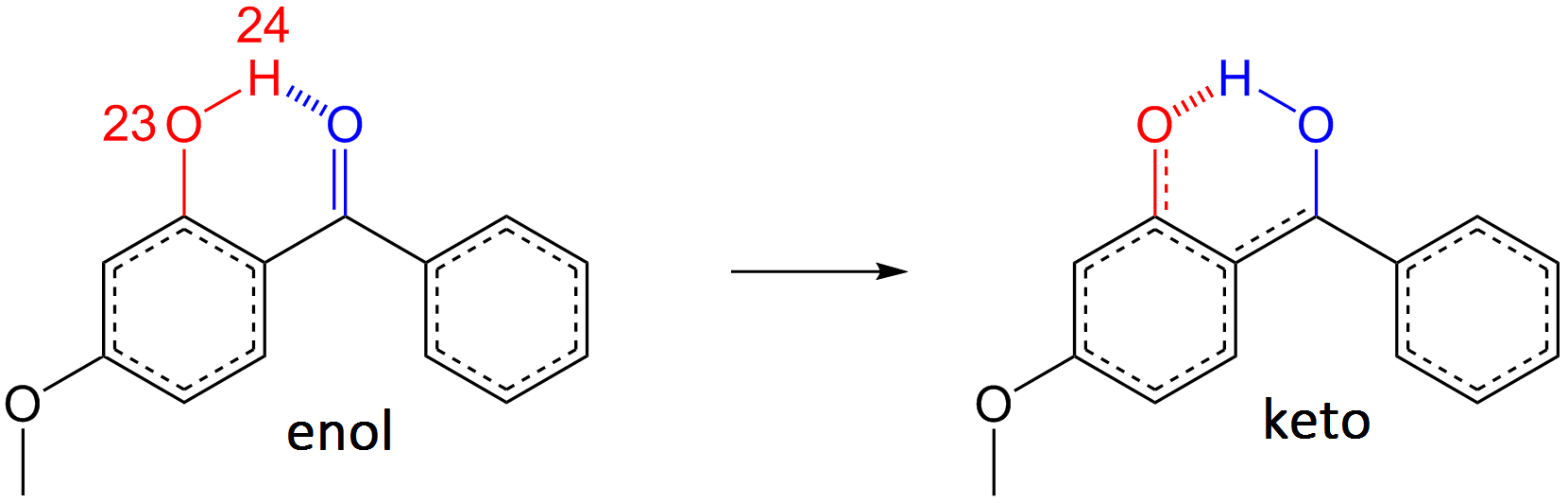
The conclusion of this study is the proposed mechanism of energy deposition:
- S0 → S2 excitation by 325 nm irradiation,
- Excited state enol → keto isomerisation and relaxation to S1: 100 fs,
- C-C bond rotation: 400 fs,
- Internal conversion from S1 → S0 through conical intersection,
- Vibrational energy transfer back to S0 enol-oxybenzone: 5 ps.
We identify a small probability for long lived photoproducts, and, with the help of ab initio calculations, we can narrow down the possibilities and suggest that a trans-keto oxybenzone isomer is the most likely photoproduct given the current study.
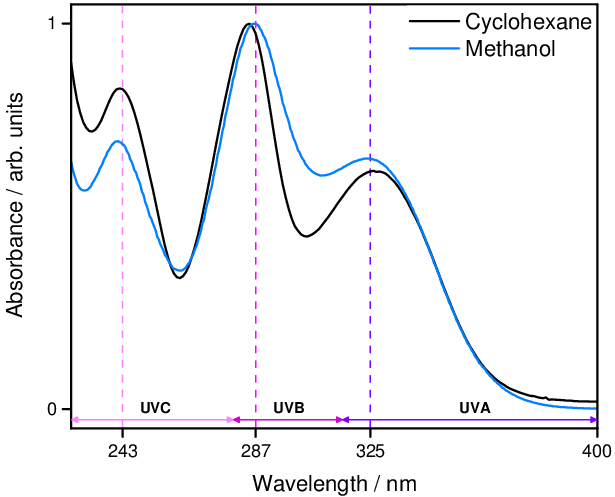
We then extended the TEAS studies to provide insight into the energy dissipation mechanism when oxybenzone is excited at its other spectra absorption maxima [2]. Of particular interest was whether UVB radiation presented any difference given that it is these wavelengths which cause photodamage in the skin. UVC radiation is another interesting consideration given industrial applications. In both cases we found that the mechanism at play appears to be the same as that in the UVA case, giving the conclusion the oxybenzone display broadband photoprotection.
You can read about this work in a summary written for Atlas of Science, What, why, how? Everyday sunscreen protection.
Sinapoyl malate
Plants also produce natural sunscreens to protect important constituents of their leaves, photosynthetic machinery for example. Sinapoyl malate has been identified as likely candidate of one such sunscreening molecule which is deposited into the upper epidermis of the leaves [3]. We applied TEAS with a range of solvents (dioxane, ACN and methanol) to understand how this molecule, along with simpler analogues sinapic acid (sinapoyl malate's precursor) and methyl sinapate (a simple derivative of sinapic acid) dissipate energy after UVB photoexcitation [4].
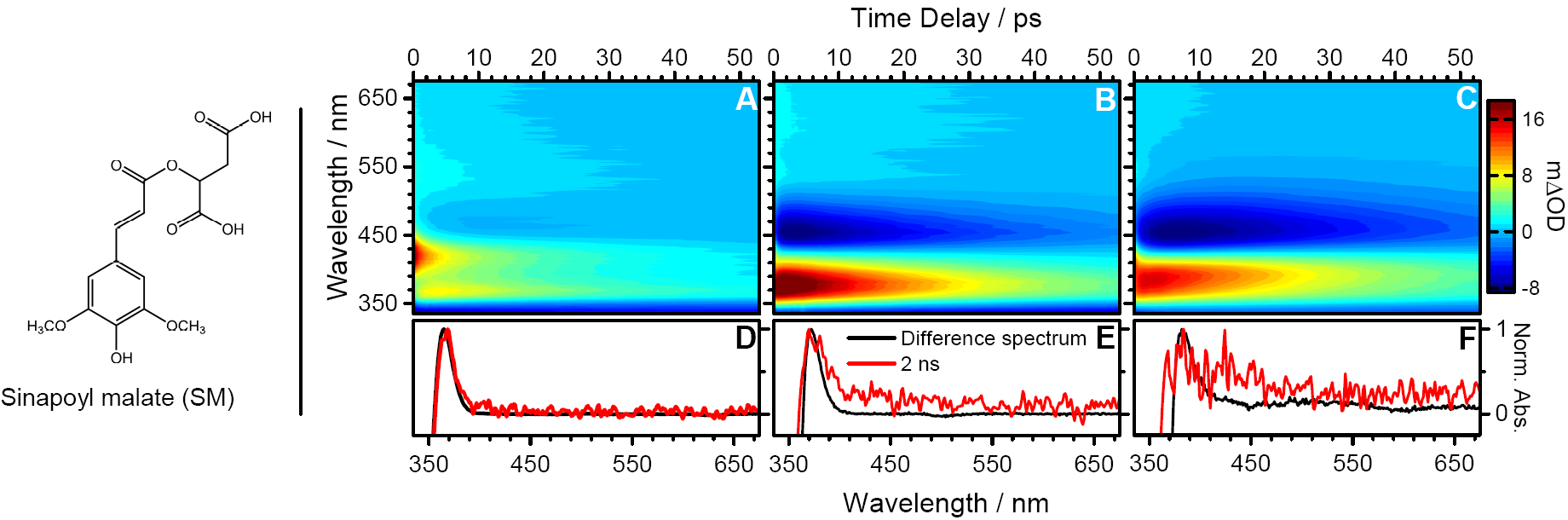
Figure: (A-C) Transient absorption spectra of sinapoyl malate in dioxane, ACN and methanol respectively. (D-F) Difference UV spectra (the difference between UV-visible spectrum before and after 10 minutes of UVB irradiation) are taken to investigate long-lived photoproducts identifying the cis isomer.
We conclude in this study that the likely dominant relaxation mechanism is nonradiative decay via a trans-cis isomerisation, repopulated the ground state, as suggested in the schematic below. The time scales involved are ultrafast, femtosecond and picosecond components are extracted through global analysis of each transient absorption spectrum.
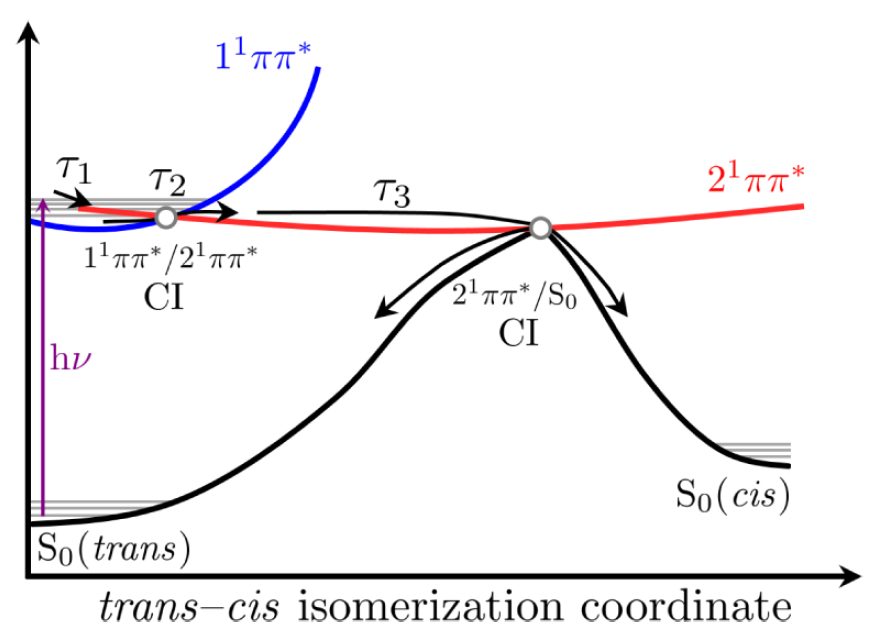
Figure: One of the relaxation mechanisms proposed in this work.
Octocrylene
This time we decided to look at one of the most popular sunscreen components, octocrylene. Here we used femtosecond pump-probe spectroscopy, as relative simple ab initio calculations to try to understand how this remarkably efficient sunscreen molecule works [5].
[1] Lewis A. Baker, Michael D. Horbury, Simon E. Greenough, Philip M. Coulter, Tolga N. V. Karsili, Gareth M. Roberts, Andrew J. Orr-Ewing, Michael N. R. Ashfold, and Vasilios G. Stavros. Probing the Ultrafast Energy Dissipation Mechanism of the Sunscreen Oxybenzone after UVA Irradiation. J. Phys. Chem. Lett., 2015, 6(8):1363–1368.
[2] Lewis A. Baker, Michael D. Horbury, Simon E. Greenough, Michael N. R. Ashfold and Vasilios G. Stavros. Broadband ultrafast photoprotection by oxybenzone across the UVB and UVC spectral regions. Photochem. Photobiol. Sci., 2015, 14(10):1814-1820.
[3] Chapple C. C. S., Vogt T., Elliis, B. E., Somerville, C. R. An Arabidopsis Mutant Defective in the General Phenylpropanoid Pathway. Plant Cell, 1992, 4, 1413−1424.
[4] Lewis A. Baker, Michael D. Horbury, Simon E. Greenough, Florent Allais, Patrick S. Walsh, Scott Habershon and Vasilios G. Stavros. Ultrafast Photoprotecting Sunscreens in Natural Plants. J. Phys. Chem. Lett., 2016, 7(1):56-61.
[5] Lewis A. Baker, Michael D. Horbury and Vasilios G. Stavros. Ultrafast photoprotective properties of the sunscreening agent octocrylene. Opt. Express, 2016, 24(10):10700-10709.
