Tania Read
About Me :
Education:
University of Warwick 2011- 2012 MSc AS:MIT
University of Plymouth 2008-2011 BSc (Hons) Analytical Chemistry Upper Second Class Honours
Contact Me:
Tania Read
University of Warwick
Gibbet Hill Road
Coventry
CV4 7AL
Welcome!
Hello, and welcome to my e-portfolio! My name is Tania and I'm currently studying on the AS:MIT course here at the University of Warwick. On this page you'll find a bit of information about me and the exciting research I'm currently working on with the Warwick Electrochemistry and Interfaces group.  Thanks for looking!
Thanks for looking!
Life Outside Warwick :
Believe it or not (and sometimes I don't) it is occasionally possible to find the time to do something completely unrelated to chemistry! On those rare occasions that I wake up and realise "Hey, I have nothing to do today", It is usually such a surprise that I am inclined to feel rather lost for things to do. When at a loss, one becomes quite inventive and as a result of this I have discovered making jewellery and baking are great ways to spend my time. However, all this baking and eating of sweet things unfortunately requires the undertaking of some form of excercise regime, to which end I will try anything fun and have recently taken up Squash. Squash is great, not only is it healthy but it's educational too - every time I play I (painfully) discover muscles I never even knew existed! Sometimes though, you wake up and all of these things just seem like too much effort; these times are perfect for just sitting back, forgetting the world and getting lost in a good book...or six.

Research :


Development and Application of Doped Diamond Electrochemical Sensors for Detection of Environmentally Relevant Species
Diamonds are a girl’s best friend:
 It is common knowledge that diamond’s physical properties make it an excellent material to work with. Famed for being the hardest natural material known to man (and also rather pretty), it is generally unreactive and thus can be used to create devices with extreme longevity. However, (yeah, there’s always a ‘but’) intrinsic diamond is also an excellent insulator – so how on earth do we make electrodes with them? That’s where Element Six come in, with some rather useful science! All of our diamond is provided for us by Element Six, who grow it using a method called Chemical Vapour Deposition (CVD). The great thing about this is they can grow exactly what we need.
It is common knowledge that diamond’s physical properties make it an excellent material to work with. Famed for being the hardest natural material known to man (and also rather pretty), it is generally unreactive and thus can be used to create devices with extreme longevity. However, (yeah, there’s always a ‘but’) intrinsic diamond is also an excellent insulator – so how on earth do we make electrodes with them? That’s where Element Six come in, with some rather useful science! All of our diamond is provided for us by Element Six, who grow it using a method called Chemical Vapour Deposition (CVD). The great thing about this is they can grow exactly what we need.Growing diamond this way allows for doping with boron; atoms of boron are substituted for carbon in the diamond crystal lattice, thus increasing the number of free charge carriers (holes) and in turn, conductivity. Depending on the ratio of boron to carbon atoms, the diamond will exhibit semi-conductor or semi-metallic conducting properties (effectively more boron = more conductive). Element Six kindly dope all of our diamond samples with boron to make it conductive. For this project I am using polycrystalline boron doped diamond (pBDD) which, due to its high boron concentration is a semi-metallic conductor, and it is also black in colour (ok, so the colour is not really relevant to the electrochemistry, but it’s cool!). Great! We have our electrochemically active diamond, so other than being hard, is it actually any better than our other metallic electrodes?
Yes! And here’s why.
Polycrystalline Boron Doped Diamond and Electrochemistry:
There are a range of properties which make pBDD an excellent electrode material; as already mentioned it’s resistive to corrosion meaning sensors can be used effectively in even harsh environments. In terms of actual electrochemistry, pBDD exhibits more useful properties such as low background currents. Perhaps even more importantly it has a wide potential window (the gap between the potentials for water reduction/oxidation) in aqueous solution, as illustrated in Figure 1; this potential window is much wider than that of other commonly used electrode materials such as platinum and gold.
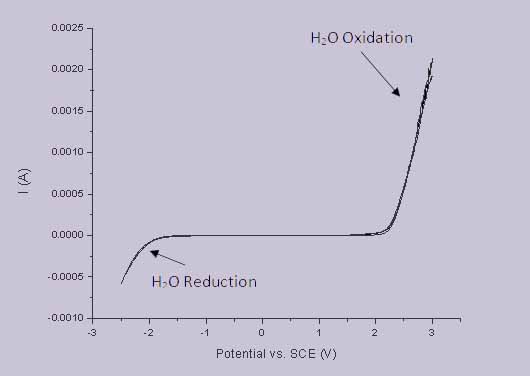
Figure 1 - Example potential window of a pBDD macro electrode.
Cyclic Voltammetry:
Cyclic voltammetry is a commonly used electrochemical technique in which the potential is swept linearly to a pre-defined value and then reversed. The current measured at the working electrode is then plotted versus potential, producing what is known as a voltammogram. Results of a cyclic voltammetry experiment are governed by the Randles-Sevcik equation and provide information on electrochemical properties of the analyte and also for characterization of electrodes. As mentioned earlier pBDD can exhibit semi-metallic properties; these can be observed in measurements with cyclic voltammetry, where for a metallic conductor the peak separation is expected to be approximately 59 mV. An example of a cyclic voltammaogram can be seen in Figure 2 (yes, it is meant to look like a duck).
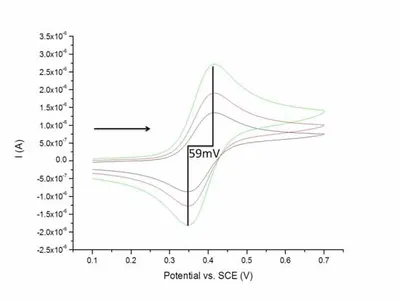
Figure 2 - Example cyclic voltammogram of a pBDD macro electrode.
My Project:
So what exactly am I doing? My project is exactly what it says on the tin, I am currently engrossed in the process of developing novel polycrystalline Boron Doped Diamond electrodes for use in the detection of environmentally relevant species. To do this I will be using a whole range of electrochemical techniques, including those mentioned above. How am I doing this? Ah, well that I won’t tell you, but I promise it’s exciting! (I get to work with diamonds every day, how could it not be?)


