Warwick scientists design model to predict cellular drug targets against Covid-19
· The covid-19 virus, like all viruses relies on their host for reproduction
· How SARS-CoV-2 captures stoichiometric amino and nucleic acids in a human lung cell has been discovered by using a computational model by scientists from the University of Warwick
· By understanding host-based metabolic perturbations inhibiting SARS-CoV-2, a rapid, experimentally testable generation of drug predictions can be made
A computational model of a human lung cell has been used to understand how SARS-CoV-2 draws on human host cell metabolism to reproduce by researchers at the University of Warwick. This study helps understand how the virus uses the host to survive, and enable drug predictions for treating the virus to be made.
Viruses rely on their host to survive, a crucial step of lifecycle is the synthesis of the virus particles within the host cell, therefore understanding this process is key to finding ways to prevent the virus from surviving.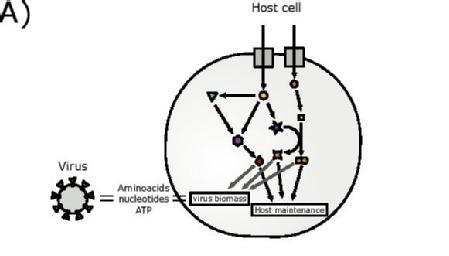
Using a computer model of a human lung cell metabolism, scientists from the School of Life Sciences at the University of Warwick have captured the stoichiometric amino and nucleic acid requirements of SARS-CoV-2, the virus that causes Covid-19. Publishing their results in the paper, ‘Inhibiting the reproduction of SARS-CoV-2 through perturbations in human lung cell metabolic network’, in the journal Life Science Alliance.
Their model has identified host-based metabolic perturbations inhibiting SARS-CoV-2 reproduction, highlighting reactions in the central metabolism, as well as amino acid and nucleotide biosynthesis pathways. In fact, researchers found that only few of these metabolic perturbations are able to selectively inhibit virus reproduction.
Researchers have also noted that some of the catalysing enzymes of such reactions have demonstrated interactions with existing drugs, which can be used for experimental testing of the presented predictions using gene knockouts and RNA-interference techniques.
Professor Orkun Soyer, from the School of Life Sciences at the University of Warwick comments:
“We have created a stoichiometric biomass function for the COVID-19-causing SARS-CoV-2 virus and incorporated this into a human lung cell genome scale metabolic model.
“We then predicted reaction perturbations that can inhibit SARS-CoV-2 reproduction in general or selectively, without inhibiting the host metabolic maintenance. The predicted reactions primarily fall onto glycolysis and oxidative phosphorylation pathways, and their connections to amino acid biosynthesis pathways.”
Dr Hadrien Delattre, from the School of Life Sciences at the University of Warwick adds:
“Together, these results highlight the possibility of targeting host metabolism for inhibition of SARS-CoV-2 reproduction in human cells in general and in human lung cells specifically.
He added, “More research needs to be carried out to explore SARS-CoV-2 infected cells and their metabolism, however the model developed here by the researchers can be used as a starting point for testing out specific drug predictions”.
ENDS
25 NOVEMBER 2020
NOTES TO EDITORS
High-res images available at:
https://warwick.ac.uk/services/communications/medialibrary/images/november_2020/delaterre_jpeg.jpg
Caption: Schematic representation of the integrated host-virus metabolic modelling approach used in the article. The biomass composition of SARS-CoV-2 is estimated based on genomic and structural informations and then embedded in the metabolic network model of the host cell. This model is then used to predict the metabolic fluxes supporting virus production and effects of perturbations.
Credit: University of Warwick
Paper available to view at: https://www.life-science-alliance.org/content/4/1/e202000869
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 7920 531 221
E-mail: alice.j.scott@warwick.ac.uk
