Modelling Methods/ Méthodes de modélisation
Extending a cost-effectiveness framework to account for elimination goals
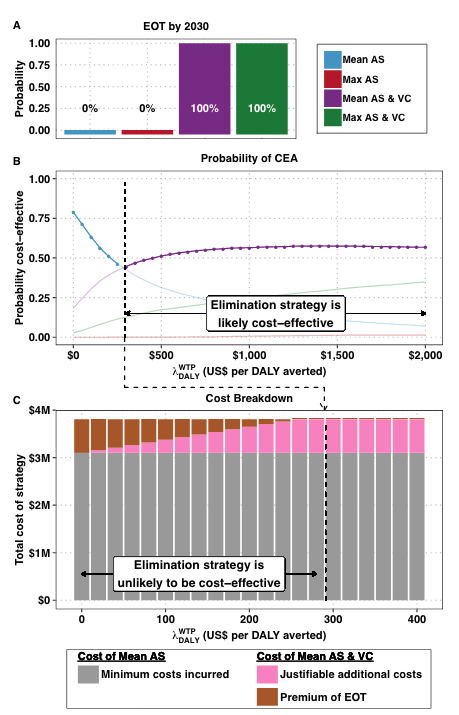
Various diseases are earmarked for elimination by the global health community. While the health economic implications of elimination have been discussed before, the combination of uncertainty, cost-effectiveness in terms of cases averted, and elimination in the face of rising per-case costs has not been tackled before.
We propose an approach that considers the tension between the dual objectives of cost-effectiveness and elimination while incorporating uncertainty in these objectives. This framework enables us to compute the additional costs of implementing a strategy which is more likely to achieve elimination beyond what would normally be considered cost-effective - which we call the premium of elimination.
We apply our method to strategies against human African trypanosomiasis in three settings, but this method could be directly applied to simulation-based studies of the cost-effectiveness of other disease elimination efforts. The method yields common metrics of efficiency for stakeholders who have different objectives.
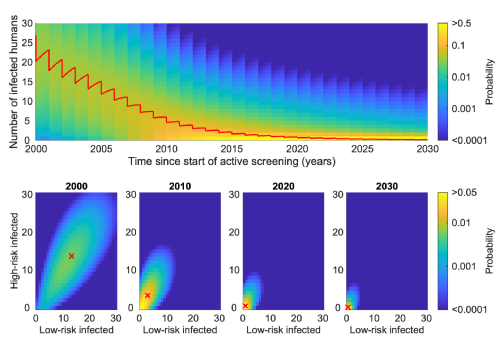
Simulating elimination dynamics in connected villages
By simplifying the infection structure of the Warwick gHAT model, we reduce the dimensionality of our infection system such that we can utilise the powerful method of Kolmogorov forward equations. This newly developed model variant captures the infection dynamics within the groups of spatially-connected villages that make up a health zone. Using this model, the probability of elimination of infection can be calculated on different spatial scales in a consistent manner and scaled between them. We see comparable results at the health zone level to the higher-dimensional models. However, the novel flexibility of the new model allows interventions to be modelled specific to each village, which introduces the modelling framework that we can use to consider the possible future strategies: test-and-treat or direct treatment of individuals living in villages where cases have been found, using new drug, acoziborole.
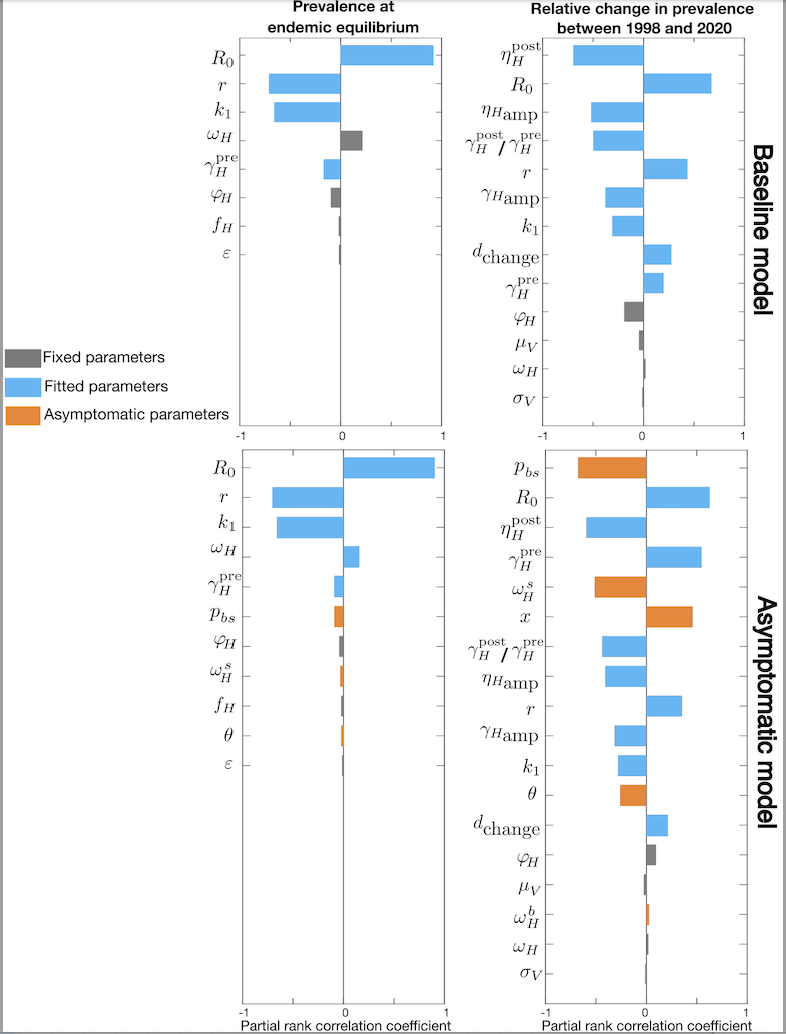
Potential impact of asymptomatic human infections on gHAT transmission
There is evidence of asymptomatic human gHAT infection but there is uncertainty around the role it plays in overall transmission and maintenance. To study this challenge, we developed a novel model by extending our established baseline framework for gHAT to account for asymptomatic infections including those in people with blood-dwelling trypanosomes, without discernible symptoms, and those with parasites only detectable in skin. Our new model adds extra parameters to the baseline model including different self-cure, recovery, transmission rate from skin-only infections, and the proportion of exposures resulting in initial skin or blood infection. Performing sensitivity analysis suggests the new parameters introduced in the asymptomatic model can impact the infection dynamics substantially. For some plausible parameterisations, an initial fall in infection prevalence - due to screening and treatment interventions - could subsequently stagnate even under continued screening due to the formation of a new, lower endemic equilibrium. Excluding this scenario, our results still highlight the possibility for asymptomatic infection to slow down progress towards elimination of transmission. Location-specific model fitting will be needed to determine if and where this could pose a threat.
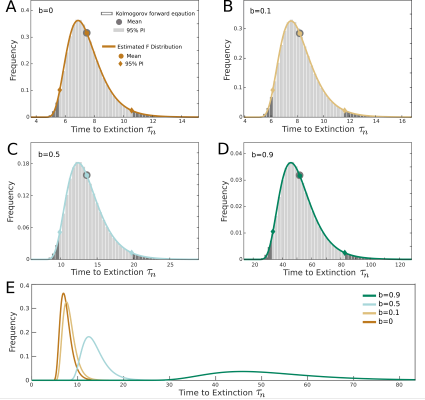
Elimination thresholds for the gHAT model
Predicting the time to elimination of gHAT transmission in different circumstances is pivotal to our research. Analysis has been undertaken to identify a threshold value for the deterministic model that equates to the mean extinction time of the associated stochastic model.
We study this problem with the help of an effective "birth-death" description of infection and recovery processes and present a practical method to estimate the distribution of extinction times. This work can be used in the future to enable more robust predictions for time to local elimination of transmission when using deterministic models.