Infection reservoirs/ Réservoirs d'infection
Will Cryptic Infections Impact the Elimination Goal?
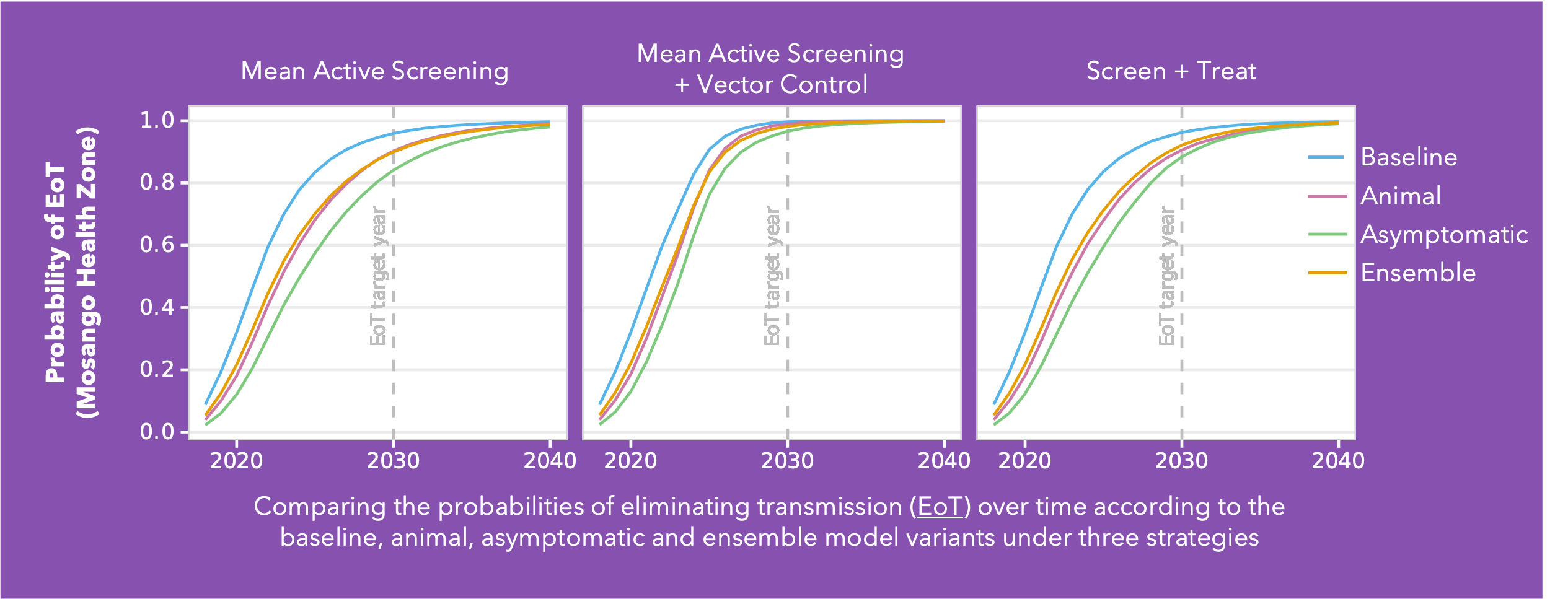
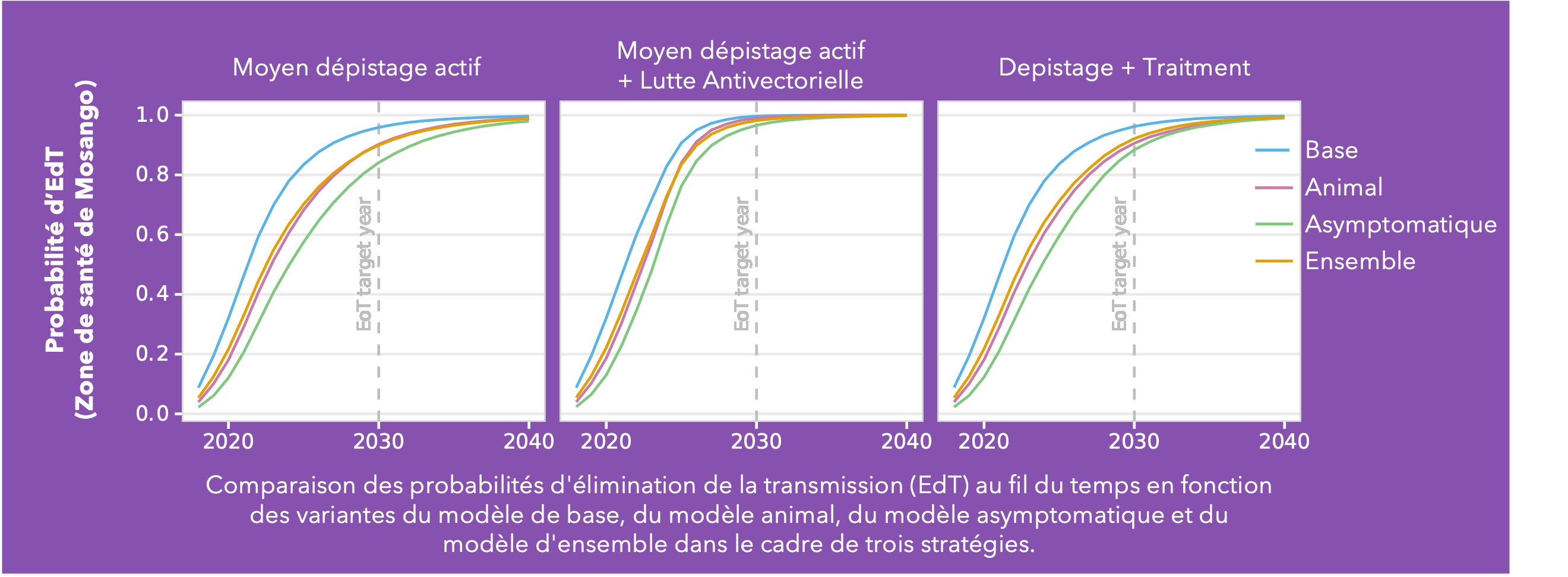
Conducted across five health zones in the DRC and spanning a broad range of prevalences, Ron’s analysis delves into the much-debated topic of cryptic infections and their potential impact on eliminating transmission (EoT). Building on the baseline Warwick gHAT model (blue line in the figure) two additional model variants were added: the animal transmission model– allowing for the potential role of animals as carriers and transmitters of infection (red line in figure) - and the asymptomatic transmission model – allowing for the presence of asymptomatic human infections, their detectability in blood tests, and their capacity to self-cure without intervention (green line in figure). By gathering statistical evidence for these model variants in each health zone, it is possible to infer which has the greatest statistical support. This ensemble of model variants (orange line in figure) was used to assess the probability of EoT across the health zones under specific strategies.
As anticipated, the baseline model is the most optimistic regarding the probability of achieving EoT. Furthermore, simulations under a Screen + Treat strategy highlight the potential benefit in reducing transmission by removing the parasitological confirmation step before treatment, particularly in the asymptomatic model variant. The results also highlight the advantages of vector control measures specifically in the presence of cryptic infections. However, whilst vector control is a valuable tool, implementing such a resource-intensive intervention necessitates careful prioritisation.
This analysis indicates we can be cautiously optimistic and that despite emerging evidence of trypanosomes in the skin without detectable blood parasites, their contribution to transmission dynamics appears limited in the DRC. Nevertheless, we acknowledge the potential for asymptomatic transmission, albeit with a relatively minor impact on our elimination targets. As we refine our understanding of gHAT transmission dynamics, these insights will serve as invaluable guides for developing targeted interventions.
Crump RE, Aliee M , Sutherland SA, Huang C, Crowley EH, Spencer SEF, Keeling MJ, Shampa C, Miaka EM and Rock KS Modelling timelines to eliminaLink opens in a new windowtion of sleeping sickness in the DRC accounting for possible cryptic human and animal transmission Link opens in a new windowMedRXiV (not yet peer-reviewed) Paper summaries: English Link opens in a new windowFrenchLink opens in a new window
Assessing evidence for animal reservoirs that contribute to transmission of gHAT in the DRC
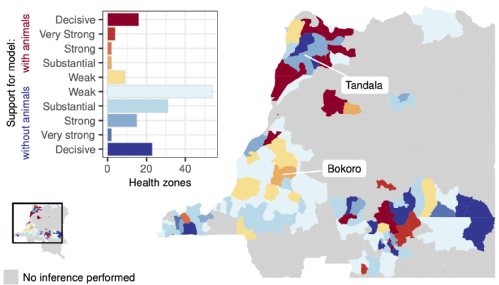
With gHAT targeted for elimination of transmission (EoT) to humans by 2030 there remain fundamental questions about the presence of non-human animal transmission cycles and their potential role in slowing progress towards, or even preventing, EoT. In this study we used two model variants, one with and one without animal transmission, to assess whether animals contribute to transmission in specific regions of the DRC, and if so, how their presence could impact the timing of EoT. We conclude that there are 24/158 health zones where there is moderate or high support for some animal transmission, however, even in these regions, we estimate that animals would be extremely unlikely to maintain transmission on their own. Animal transmission could hamper progress towards EoT in some settings, with projections under continuing interventions indicating that the number of health zones expected to achieve EoT by 2030 reduces from 68 to 61 if animals are included in the model. With supplementary vector control added to medical screening and treatment interventions, the expected number of health zones meeting the goal increases to 147 for the model including animals due to the impact of vector reduction on transmission to and from all hosts.
Click here to view our infographic.
Potential impact of asymptomatic human infections on gHAT transmission
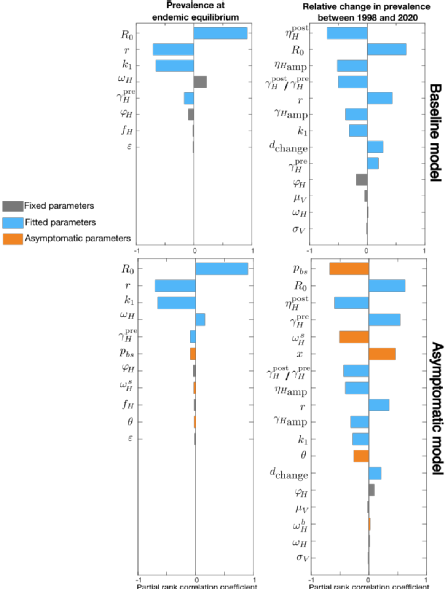
There is evidence of asymptomatic human gHAT infection but there is uncertainty around the role it plays in overall transmission and maintenance. To study this challenge, we developed a novel model by extending our established baseline framework for gHAT to account for asymptomatic infections including those in people with blood-dwelling trypanosomes, without discernible symptoms, and those with parasites only detectable in skin. Our new model adds extra parameters to the baseline model including different self-cure, recovery, transmission rate from skin-only infections, and the proportion of exposures resulting in initial skin or blood infection. Performing sensitivity analysis suggests the new parameters introduced in the asymptomatic model can impact the infection dynamics substantially. For some plausible parameterisations, an initial fall in infection prevalence - due to screening and treatment interventions - could subsequently stagnate even under continued screening due to the formation of a new, lower endemic equilibrium. Excluding this scenario, our results still highlight the possibility for asymptomatic infection to slow down progress towards elimination of transmission. Location-specific model fitting will be needed to determine if and where this could pose a threat.