Research
Petroleomics
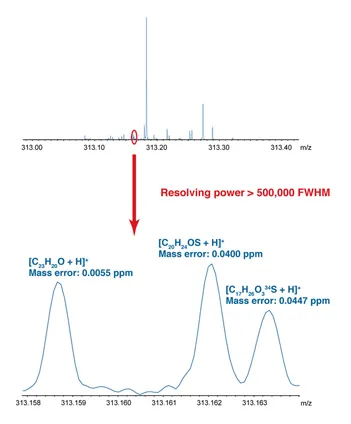
Petroleum-related samples, such as crude oils, are highly complex mixtures and typically contain tens of thouands of components. The characterization of such mixtures using mass spectrometry has been termed "petroleomics," and is a challenging area of research. Ultrahigh resolving power and sub-ppm mass accuracy are required for the resolution and assignment of the many thousands of compounds present. Fourier transform ion cyclotron resonance (FTICR) mass spectrometry represents the state-of-the-art with respect to such performance characteristics and so is uniquely suited to the study of complex mixtures.
For petroleum-related research at the University of Warwick, a 12 T solariX (Bruker Daltonics) FTICR mass spectrometer is used, with access to a diverse variety of ionization methods, dissociation techniques, and chromatographic instrumentation. A wide range of petroleum-related samples can be analyzed, including crude oils, oil extracts, and fuels, amongst others. Electrospray ionization (ESI), or a derivative such as nanospray, and atmospheric pressure photoionization (APPI) are typically used as the ionization methods of first choice, depending upon requirements. Both techniques are relatively soft ionization methods and thus minimize fragmentation. ESI is more suitable for ionic and highly polar compounds, while APPI can be used for characterizing less polar compounds which are not observed using ESI, such as polyaromatic hydrocarbons and thiophenes, amongst others.
Once the components of a petroleum sample can be resolved and the molecular compositions can be assigned, the data can be categorized according to heteroatom content, carbon number, and double bond equivalents (DBE) or hydrogen deficiency (Z). A variety of data visualization methods can be used to represent the profile of a sample and samples can then be compared. This allows the effects of anthropogenic and natural processes to be monitored. An example of such work at the University of Warwick is the study of the effects of simulated sunlight on crude oil, where it was determined that particular compound classes, such as sulfur-containing species, were more prone to photooxidation and that molecules of lower DBE values were amongst the most reactive. It is expected that the findings will contribute to an understanding of the behavior of petroleum in the environment, for example.
Naphthenic acids
Naphthenic acids are one such class of compound found within crude oils and have the empirical formula: CnH2n+ZO2, where Z is a negative value and is frequently referred to as the "hydrogen deficiency." Such species can cause corrosion in refinery equipment, resulting in costs that are ultimately passed on to the consumer, and the corrosiveness of the acids is believed to be linked to their size and structure.
The total acid number (TAN) of an oil, defined as the number of milligrams of potassium hydroxide required to neutralize one gram of crude oil, has always been believed to be indicative of the naphthenic acid content, but it is increasingly being accepted that the TAN of an oil is not such a reliable guide. Development of methods for characterizing the naphthenic acid content of crude oils is therefore highly desirable. Work performed at the University of Warwick has demonstrated the naphthenic acid profiles can act as fingerprints for different oilfields.
In addition to the potential corrosiveness, naphthenic acids are also known to be toxic to a range of aquatic organisms. As a result, naphthenic acids are of particular concern for both the petroleum industry and environmental regulatory organizations.
Athabasca oil sands
The Athabasca oil sands in Canada and represent a less conventional source of petroleum and are estimated to be able to provide 174 billion barrels of bitumen using current technology. These oil sands are a mixture of clay, water, sand, and bitumen, where the bitumen is extracted from the deposits and then "upgraded" to synthetic oil. During the extraction process, large quantities of water are involved: approximately three barrels of water are required to produce one barrel of oil from the oil sands. The water, known as oil sands process water (OSPW), can be recycled and is stored in large "tailings ponds." There is a need to study oil sands process water to determine which petroleum-related components may be entering the system and thus potentially enter the wider environment.
The research community has often focused upon the naphthenic acids from the oil sands, but work at the University of Warwick has demonstrated that less polar components are also present and that a wider range of compounds now needs to be monitored. In addition, it has been found that water samples of environmental and industrial origins can be differentiated and, furthermore, that samples taken from different companies in the same oil sands producing region can be distinguished.