Membrane Protein Folding and Interactions
Developing methods for describing the assembly, interactions and three-dimensional structures of membrane proteins is the main focus of work in the lab. We use a wide-range of biochemical, computational, and biophysical techniques, including solution-state NMR spectroscopy. Of particular interest are membrane proteins involved in immune response and cancer developement.
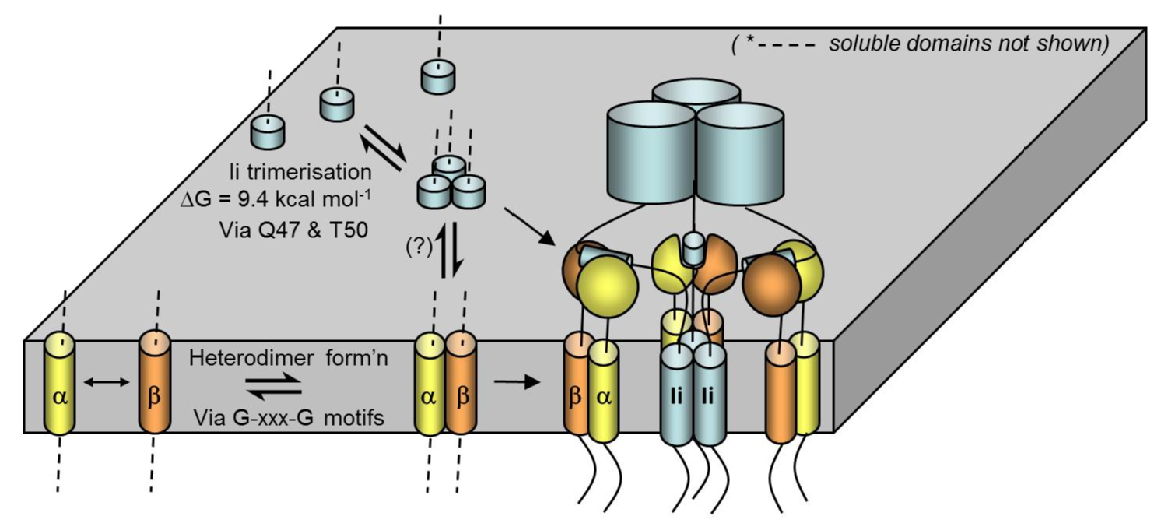
Figure: Schematic summarizing the roles of TM domains in the assembly of the Class II Major Histocompatibility complex, as described in three of our papers below:
- Dixon, A.M., Drake, L., Hughes, K.T., Sargent, E., Hunt, D., Harton, J.A., Drake, J.R. Differential Transmembrane Domain GxxxG Motif Pairing Impacts MHC Class II Structure, J. Biol. Chem., 2014, 289, p. 11695-703
- King, G. and Dixon, A.M. Evidence for Role of Transmembrane Helix-Helix Interactions in the Assembly of the Class II Major Histocompatibility Complex, Molecular BioSystems, 2010, 6, p. 1650-1661
- Dixon, A. M., Stanley B.J., Matthews, E.E., Dawson, J.P., Engelman, D.M., Invariant chain transmembrane domain trimerization: A step in MHC class II assembly, Biochemistry, 2006, 45, p. 5228-5234.
More generally, we are also interested in how protein-protein and protein-lipid interactions occur in membrane bilayers, and what molecular properties control these interactions. The papers below summarize some of our recent work in these areas.
- Breeze, E., Dzimitrowicz, N., Kriechbaumer, V., Brooks, R., Botchway, S.W., Brady, J.P., Hawes, C., Dixon, A.M., Schnell, J.R., Fricker, M.D., Frigerio, L., A C-terminal amphipathic helix is necessary for the in vivo tubule-shaping function of a plant reticulon, Proc. Natl. Acad. Sci., 2016, 113, 10902-10907.
- Lock, A., Forfar, R., Weston, C., Bowsher, L., Upton, G.J.G., Reynolds, C.A., Ladds, G., Dixon, A.M., One Motif to Bind Them: A Small-XXX-Small Motif Affects Transmembrane Domain 1 Oligomerization, Function, Localization, and Cross-talk Between Two Yeast GPCRs, Biochimica et Biophysica Acta – Biomembranes, 2014, 1838, p. 3036-3051.
- Beevers, A.J., Nash, A., Salazar-Cancino, M., Scott, D.J., Notman, R., and Dixon, A.M., Effects of the Oncogenic V664E Mutation on Membrane Insertion, Structure, and Sequence-dependent Interactions of the Neu Transmembrane Domain in Micelles and Model Memebranes: An Integrated Biophysical and Simulation Study, Biochemistry, 2012, 51, p. 2558-2568.
- Jenei, Z.A., Warren, G.Z.L., Hasan, M., Zammit, V.A. and Dixon, A.M. Packing of Transmembrane Domain 2 of Carnitine Palmitoyltransferase-1A affects Oligomerization and Malonyl-CoA Sensitivity of the Mitochondrial Outer Membrane Protein, FASEB J, 2011, 25, p. 4522-4530.
- King, G., Oates, J., Patel, D., van den Berg, H., and Dixon, A.M., Towards a Structural Understanding of the Smallest Known Oncoprotein: Investigation of the Bovine Papillomavirus E5 Protein using Solution-state NMR, BBA - Biomembranes, 2011, 1808, p. 1493-1501.
