Membrane Curvature
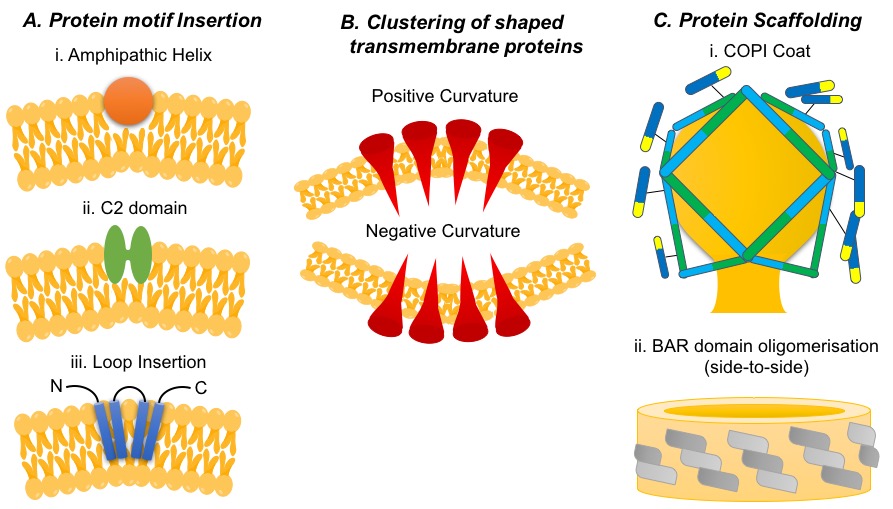
Biological processes at cellular membranes are modulated by the physical properties of the membrane itself; one of which is membrane-curvature. And whilst lipid composition does play a role, it is largely dependent on the behaviour of specific proteins. Generating, sensing and maintaining membrane-curvature is an active process mediated by specialised proteins. Several mechanisms for these kinds of behaviour have been identified, some of which are shown in Figure 1. However, due to the universality within eukaryotic cells, it is likely many more exist that are yet to be discovered. One well known example is that of Clathrin mediated endocytosis; Clathrin is a coat protein that assists in the inward budding of the plasma membrane, widely used for the uptake of substances needed by the cell.
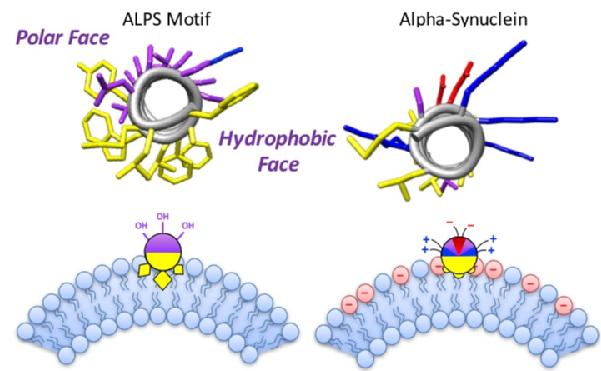
Bacteria and eukaryotic cells contain geometry-sensing proteins within their cytosol that are able to recognise the curvature of lipid membranes. Membrane curvature sensors have been linked to a diverse range of cellular processes, such as lipid transfer between membranes, the tethering of vesicles at the Golgi apparatus, and the assembly-disassembly cycle of protein coats. However, whilst an increasing number of membrane-surface associated proteins have been identified as sensors of membrane curvature, the mechanisms behind curvature sensing are still not completely understood and can differ greatly between different protein families. Sensors that have previously been identified include long amphipathic helices (APHs) such as that found in ArfGAP1, a special type of APH called amphipathic lipid-packing sensors (ALPS), as well as the N-terminal region of α-synuclein. Although these are both APHs, they have remarkably different chemistries; ALPS motifs lack charged residues in their polar face, whereas the APH in α-synuclein does not contain large bulky hydrophobic residues but does have a very well-defined zwitterionic polar face. This is shown in Figure 2.
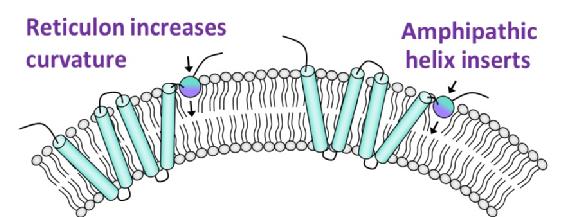
The reticulons (RTN) are a large family of integral membrane proteins, primarily located in the endoplasmic reticulum (ER) and conserved across all eukaryotes. They are predominantly responsible for maintaining the high curvature required for the formation of ER tubules and mutations in these proteins lead to motor neuron diseases. Recently, we have identified a putative C-terminal amphipathic helix (APH) in RTNLB13, one of the smallest RTN isoforms in Arabidopsis thaliana, which is necessary for the proteins function. This APH region is considered to be conserved throughout the RTN family, representing a key structural requirement in this class of proteins. It is therefore of interest to explore the mechanism via which these APHs are recognizing and/or stabilising the curvature found in ER membrane tubules.
Relevant publications:
Brooks, R.L., Mistry, C.S. & Dixon, A.M., Curvature sensing amphipathic helix in the C-terminus of RTNLB13 is conserved in all endoplasmic reticulum shaping reticulons in Arabidopsis thaliana. Sci. Rep., 2021, 11, 6326.
Brooks, R.L. and Dixon, A.M., Revealing the Mechanism of Protein-Lipid Interactions for a Putative Membrane Curvature Sensor in Plant Endoplasmic Reticulum. Biochim. Biophys. Acta. Biomem,, 2020, 1862, 183160.
Chow, M., Sklepari, M., Frigerio, L. and Dixon, A.M., Bacterial expression, purification and biophysical characterization of the smallest plant reticulon isoform, RTNLB13, Prot. Expr. Purif., 2018, 152, 31-39.
Breeze, E., Dzimitrowicz, N., Kriechbaumer, V., Brooks, R., Botchway, S.W., Brady, J.P., Hawes, C., Dixon, A.M., Schnell, J.R., Fricker, M.D., Frigerio, L., A C-terminal amphipathic helix is necessary for the in vivo tubule-shaping function of a plant reticulon, Proc. Natl. Acad. Sci., 2016, 113, 10902-10907.
