Peptide Modification and Antimicrobial Peptides
Peptide-based drugs represent a significant portion of total pharmaceutical sales (estimated to exceed $70 billion in 2019). However, they are readily degraded in the bloodstream, which is problematic for a drug molecule. In order to improve their resistance to enzymatic degradation, and to enhance their physicochemical properties, peptides are often modified or cyclised instead. In fact, the best examples of peptide drugs are cyclic - both insulin (used for treatment of type II diabetes) and cyclosporine A (an immunosuppressant that has revolutionised human transplant therapy) are cyclic peptide drugs. Cyclosporine A is also modified (N-methylation) and contains non-standard amino acids, which improve its absorption and other properties.
Oxetanes are useful molecules in medicinal chemistry, and oxetane-modified peptides have been shown to have increased efficiency of cyclisation. We think that oxetane modification is able to change the 3D structure of peptides, without reducing their biological activity.
Antimicrobial peptides are another exciting avenue of peptide drug research. With antibiotic resistance as one of the biggest threats to global health today, it is vital that we find alternative treatments. Antimicrobial peptides represent a diverse class of natural and synthetic peptides which demonstrate activity against microbes. We are using screening a selection of synthetic peptides inspired by nature, to investigate how changing sequence, cyclising and modifying antimicrobial peptides affects their biological activity.
Relevant publications:
Craik, D. J., Fairlie, D. P., Liras, S. and Price, D., 2013. The Future of Peptide-based Drugs. Chemical Biology and Drug Design, 81(1), pp.136-147.
Henninot, A., Collins, J. C. and Nuss, J. M., 2017. The Current State of Peptide Drug Discovery: Back to the Future? Journal of Medicinal Chemistry, 61, pp.1382–1414.
Ito, K., Passioura, T. and Suga, H., 2013. Technologies for the synthesis of mRNA-encoding libraries and discovery of bioactive natural product-inspired non-traditional macrocyclic peptides. Molecules, 18(3), pp.3502-3528.
https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
Li, J., Koh, J.J., Liu, S., Lakshminarayanan, R., Verma, C.S. and Beuerman, R.W., 2017. Membrane active antimicrobial peptides: translating mechanistic insights to design. Frontiers in neuroscience, 11, p.73.
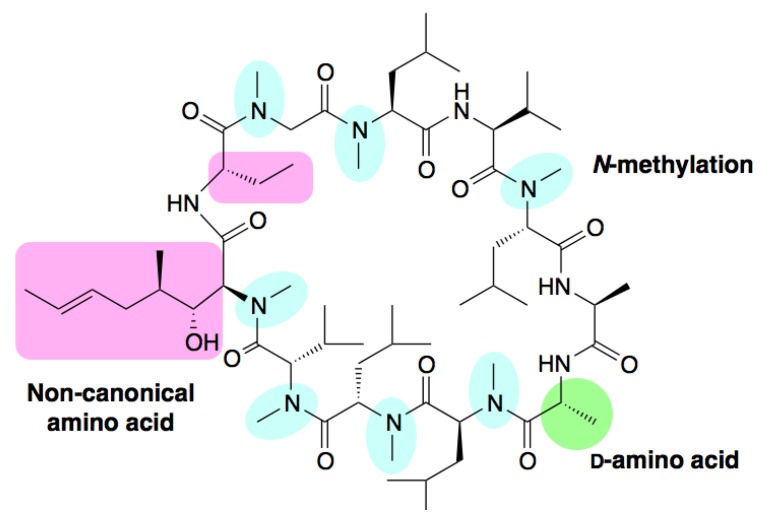
Structure of cyclosporine A (reproduced from Ito et al., 2013)
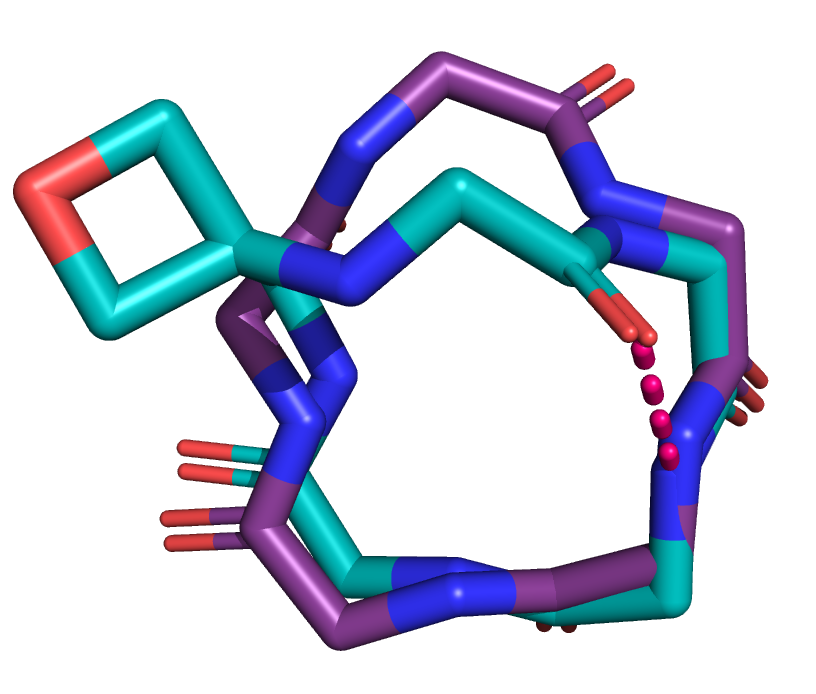
Oxetane modification changes the structure of the backbone of a cyclic peptide
