Adam Cowden
"In the MSc year (1+3) I completed two projects on the isolation of natural products: firstly, a project on carbohydrates from wood and plant material and secondly, a family of molecules called mycosporine-like amino acids extracted from marine algae. I chose to continue the latter project into a PhD, inspired by mycosporine-like amino acids from red seaweed and cyanobacteria to synthesise molecules with similar properties and to unravel their unique photodynamics with ultrafast laser spectroscopy. It appears that MAAs are nature's own sunscreen of choice."
MSc Mini-Project 1 (May-June 2019): Enzymatic Degradation Products of Lignin by FT-ICR Mass Spectrometry
The aim of my first MSc project was to evaluate whether the activity of an enzyme acting on a complex natural substrate can be detected via ultrahigh resolution mass spectrometry (FT-ICR MS). To do this I investigated the microbial conversion of lignin residue that had been extracted from plant cells. The oxidative depolymerisation of lignocellulosic biomass is thought to offer the best renewable feedstock for aromatic carbon molecules and is arguably the only real alternative to oil refining for some high value chemicals. Lignin extracts were incubated with five known bacterial lignocellulose-active enzymes and low molecular weight aromatic products were observed by reverse phase liquid chromatography (RP-HPLC). My final report concluded that direct infusion FT-ICR MS in negative ion detection mode is a suitable technique to monitor the intermediate oligomeric products formed during lignin enzymatic breakdown. The identification of degradation products from the transformation of polymeric lignin and an understanding of the substrate specificity of particular enzymes will help to decipher the metabolic pathways for microbial lignin breakdown, improve our knowledge of the enzymology of native lignin metabolism and inform biocatalytic lignin valorisation strategies.
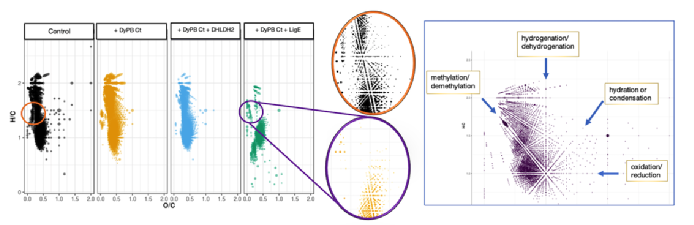
(Left) Van Krevelen diagram of GreenValue® lignin extracts after three different enzymatic digests. (Middle) Detail comparing the control (without enzyme, in orange) with the digest (with a two-enzyme combination, in purple). (Right) Annotated van Krevelen diagram generated from a generic organosolv lignin control. Annotations record the transformations in chemical space that links the identified compounds and assignments are confirmed by extrapolating to the intercept with either axis.
MSc Mini-Project 2 (July-September 2019): Mycosporines: Microbial Sunscreens
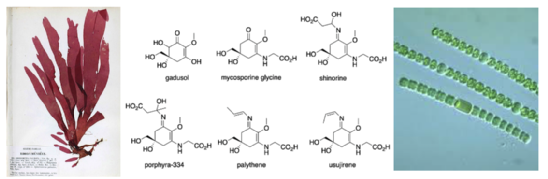
A selection of mycosporine derivatives that have been found in marine species (including marine algae and cyanobacteria)
PhD 2019-2023
I am currently investigating mycosporines and their mycosporine-like amino acid derivatives, MAAs. These are a family of structurally and chemically related compounds that absorb radiation from the solar ultraviolet radiation (UVR) spectrum and follow fast relaxation dynamics without losing UV absorbing power; at least in vitro. Exemplary in their class, the pair of conformational isomers, usujirene and palythene, are algal imino-mycosporines with optical density maxima at wavelengths 357 and 360 nm respectively; which are useful parameters for UVA absorbers. Computational studies using time-dependent density functional theory (TD-DFT) predict that these potential sunscreen molecules can rapidly interconvert following excitation, perhaps explaining the very efficient non-radiative dispersion of excited state energy.
In addition, a cluster of four genes (Ava_3855-3858) identified in cyanobacteria encodes enzymes that can convert sedoheptulose-7-phosphate (7-SHP) to the di-substituted imino-MAA, shinorine. As a group, we aim to extract and purify these and other molecules of interest from species of seaweed, like Palmaria palmata, and/or from recombinant E. coli cultures using flash chromatographic methods. This study will also provide synthetic precursors for the the chemo-enzymatic synthesis under investigation by the Corre Group in the School of Life Sciences (SLS, Warwick) with an aim to produce novel, promising UV filters.
Eventually we hope to be able to produce a wide range of synthetic analogues with adjusted properties in collaboration with the Wills Group in the School of Chemistry, also at Warwick and the Sampedro/Los Santos groups at the University of La Rioja, Spain. All of the syntheses are accompanied by expertise in time-resolved ultrafast laser spectroscopy at the Warwick Centre for Ultrafast Spectroscopy (WCUS) to monitor the events in the excited state following UV irradiation. With a resolution of around 20 femtoseconds we can begin to monitor directly for the first time the super fast photodynamics of these molecules.
Link to Stavros Group (PI: Prof. Vas Stavros)Link opens in a new window
Link to Corre Group site (PI: Dr. Christophe Corre)Link opens in a new window
Link to Wills Group site (PI: Prof. Martin Wills)Link opens in a new window
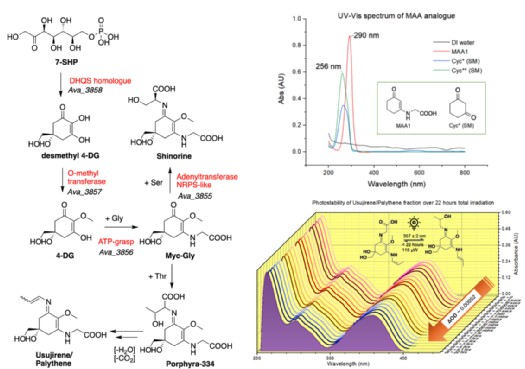
(Left) Genes and enzymes of the biosynthetic pathway to some MAAs (Bottom right) steady-state photo-stability studies using, and (Top right) UV spectra obtained of the first synthetic analogue made in the lab
Previous experience:
MChemX (Hons) from the University of Edinburgh (Sep 2013- July 2018)
I completed my undergraduate Masters in Chemistry with a Year Abroad at Edinburgh University. My final year courses included: Properties and Reactions of Matter, Bio-macromolecules, Advanced Organic Synthesis, Chemical Medicine, and Techniques and Concepts in Inorganic Chemistry. Key modules and skills that have aided my current research: pericyclic reactions, organometallic compounds, solid-phase peptide synthesis, carbon-ligand multi-bonding, small molecule activation, natural product synthesis, metals in medicine, protein-protein interactions, molecular magnetism, reaction dynamics and cyclic voltammetry. While my degree equipped me for a range of paths my major interest going forward is in synthetic organic chemistry and molecular biology.
The scope of my final-year Honours project (2017/18) was to improve the synthesis of key monomers with amine (Troeger's Base-type) linkers. Previous reaction schemes relied on time-consuming separations and produced the monomer in poor yield. The polymeric product of the scheme has been proposed as a permeable and selective membrane for gas separation and/or storage material that was able to exceed the then current state-of-the-art Robeson limit (2008).
Research placement (Year 4), the University of Bologna, Italy:
I undertook research at the Department of Chemistry "Giacomo Ciamician", leading to my first publication, in open-access journal Biomimetics, of a paper entitled "The Antioxidant Activity of Quercetin in Water Solution". The antioxidant activity of naturally occurring quercetin was investigated by combining differential oxygen-uptake kinetic measurements and B3LYP/6311+g (d,p) calculations. The results helped to rationalise quercetin’s reactivity with peroxyl radicals and its importance as a nutritional antioxidant in vivo and for biomimetic applications. Link to paper: Biomimetics 2017, 2(3), 9; https://doi.org/10.3390/biomimetics2030009Link opens in a new window