Frequently asked Questions
Key FAQs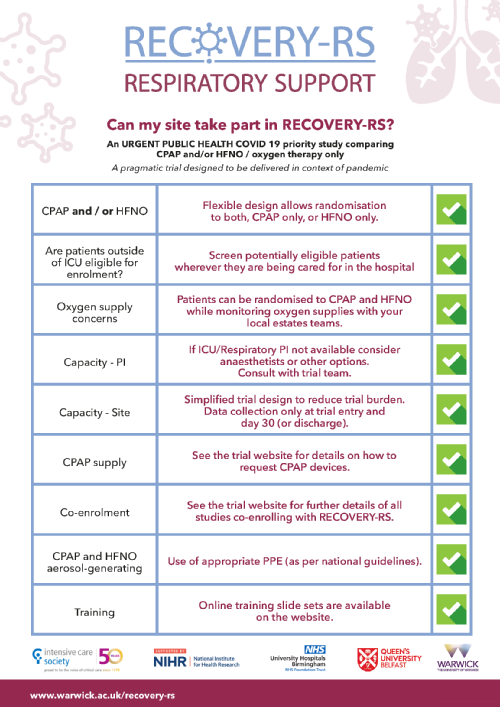
General
Equipoise
Contracting
Site set up
Screening
Eligibility
Receiving Consent
Randomisation
Co-enrolment
Treatment
Data Collection
Key FAQs |
|
|
WHAT IS THE NEED FOR THE TRIAL? |
The RECOVERY-RS trial is comparing CPAP and / or HFNO with oxygen therapy only. The rationale for the trial is that there is insufficient evidence to know which is the most effective approach. Whilst some studies have found CPAP/HFNO may improve short term outcomes, others have found evidence of harm (potentially through delayed intubation and / or ventilator associated lung injury).
The uncertainty in the evidence has led to marked variation in UK and international guidelines and clinical practice in relation to use of CPAP / HFNO and early invasive ventilation. The lack of definitive evidence and variation in practice formed the basis for the CMO’s commissioning RECOVERY-RS. |
|
SITE ACTIVATION PROCESS? |
The following is required to activate a site:
After receiving these documents Warwick CTU will:
|
|
EQUIPOISE: |
There is uncertainty regarding the benefits and harms of both CPAP and HFNO. This is reflected in conflicting international guidelines on their use. Harm may be caused if use of CPAP or HFNO delays, rather than avoids, tracheal intubation. CPAP may also cause lung injury due to large tidal volumes when used in spontaneously breathing patients with compliant lungs. |
|
TREATMENT CROSSOVER ALLOWED? |
For patients in the oxygen therapy only, CPAP or HFNO should not be routinely used. We understand that there may be patient specific reasons why a clinician has to go off protocol and provide CPAP / HFNO. This should be on a case by case basis rather than a local protocol which puts all patients on CPAP/HFNO if the oxygen therapy only arm fails. The protocol guides that treatment failures should progress to intubation and invasive ventilation. If a patient temporarily switches to a different form of treatment (e.g. for eating or drinking), this does not need to be recorded on the CRFs and does not class as a treatment crossover. |
|
HFNO/CPAP ALREADY STANDARD CARE AT YOUR SITE: |
These sites can take part but will need to follow protocol for recruited patients. |
|
HFNO CONSIDERATIONS: |
The trial team are aware of the technical issues sites may have in general with oxygen supply and the increased use of high flow devices. We recommend sites follow the advice issued in the statement from the Intensive Care Society to liaise with their engineering team on this issue. Site teams should ensure that hospital policy is followed in relation to where patients allocated to HFNO and CPAP are cared for, and that appropriate personal protective equipment is worn. If sites cannot, or do not wish to, provide HFNO, it is still possible to participate. In this situation, the randomisation system will only allocate either the standard care or CPAP arm. |
|
OXYGEN SUPPLY CONSIDERATIONS: |
We are very much aware of the technical issues sites may have in general with the increase use of high flow devices, and are reiterating the advice circulated in the ICS statement around local sites ensuring close liaison between clinical and engineering teams on this issue, (https://www.ics.ac.uk/ICS/ICS/Pdfs/COVID-19/COVID-19_Safety_Alerts/Safety_Alert_High_Flow_Oxygen.aspx) |
|
ICU/RESPIRATORY – PATIENT IDENTIFICATION: |
Screening is not limited just to patients based in ICU and can include other areas where the interventions are delivered. All patients admitted to hospital with suspected or confirmed COVID-19 can be screened for participation in the RECOVERY-RS trial. Site staff should liaise with and clinical teams across the hospital to identify patients. |
General |
|
|
Are suspected and confirmed COVID-19 cases included in the 4002 recruitment target? |
Yes |
Equipoise |
|
|
What is the argument for not using CPAP and HFNO? |
There is uncertainty regarding the benefits and harms of both CPAP and HFNO. This is reflected in conflicting international guidelines on their use. Harm may be caused if use of CPAP or HFNO delays, rather than avoids, tracheal intubation. CPAP may also cause lung injury due to large tidal volumes when used in spontaneously breathing patients with compliant lungs. |
|
How is crossover treatment defined and captured for the trial? |
25/11/2020: The following scenarios are exempt from crossover of trial intervention and going forward should not be reported on the e-CRF:
To note, expert consensus is to not use CPAP or HFNO for a second episode of non-invasive support. Should go straight to intubation. |
Contracting |
|
|
Can we negotiate terms of this contract? |
No. This is the model non-commercial agreement (mNCA) and there is no capacity to negotiate. |
|
How do we execute the contract? |
A word version of the mNCA is provided on the Site Set Up (hyperlink) page. This has been pre signed by the Sponsor. All site specific sections are editable. Please download, complete the relevant section and sign and return a fully executed version of the contract to RECOVERY-RS@warwick.ac.uk. |
|
Do you require a wet ink signature? |
Due to the current circumstances an electronic signature can be used. |
|
Does the PI declaration need to be signed? |
Yes. However, an electronic signature or email approval is sufficient. |
|
Can we amend the recruitment target in the mNCA? |
No. However, the CRN have confirmed sites will not be penalised where recruitment targets are not met. The national coordinating centre will amend targets as appropriate at a later date. Please see a letter from the NIHR Clinical Research Network Coordinating Centre to confirm this on the site set up page. |
Site set up |
|
|
What is the site activation process?
|
The following is required to activate a site: · Capacity and Capability confirmation · Site Agreement signed by all parties · Evidence of PI training
After receiving these documents Warwick CTU will: · Issue SIV and greenlight activation letter to site to open them to recruitment · Issue database log in and IVR PIN number for randomisation to site |
|
Is CAG required for RECOVERY-RS? |
As per HRA advice (available online):
Using patient data Ordinarily, applications are made for support from the Confidentiality Advisory Group (CAG) where confidential patient information is to be processed without consent for research and non-research activities. A temporary arrangement has been made for COVID-19 studies going through the fast-track review process, in which support from CAG is not required. However, CAG is providing advice as part of the fast-track process. For more information about this process, read our guidance for using patient data. (https://www.hra.nhs.uk/covid-19-research/)
The HRA approval for RECOVERY-RS is confirmation that the trial has been reviewed by HRA and CAG and approved not requiring CAG approval. |
|
If standard care already includes high flow/CPAP can a site still take part? |
These sites can take part but will need to follow protocol for recruited patients. |
|
Do the trial documents (PIS, consent forms etc.) need to be localised? |
We have amended these documents so that they no longer require updating. Current versions are available from the site set up page. |
|
Please can you advise on whether the study team would require CV and GCP? |
We are not collecting CVs or GCP for any of the study team. We do not mandate GCP however optional targeted GCP is available on the trial website.
The HRA states: Members of the research team in such studies are expected to be qualified by education, training or experience but should not be required or expected to undertake GCP training.
The RECOVERY-RS protocol and trial processes have been designed to ensure the RECOVERY-RS trial is conducted in a manner that protects the rights, safety and wellbeing of research participants and that research data are reliable. |
|
Can our site participate if we have concerns over the use of HFNO in COVID-19 patients? |
The trial team are aware of the technical issues sites may have in general with oxygen supply and the increased use of high flow devices. We recommend sites follow the advice issued in the statement from the Intensive Care Society to liaise with their engineering team on this issue. Site teams should ensure that hospital policy is followed in relation to where patients allocated to HFNO and CPAP are cared for, and that appropriate personal protective equipment is worn. If sites cannot, or do not wish to, provide HFNO, it is still possible to participate. In this situation, the randomisation system will only allocate either the standard care or CPAP arm. |
|
What are the minimum training requirements? |
As a minimum the PI must have completed SIV training or read all of the training slide sets available on the training page of the website in order for green light to be issued. It is the PI's responsibility to delegate trial roles within the site team. The full SIV slides are available on the website, in addition to reduced slide sets relevant to different trial roles. All site staff must complete relevant training to their trial role prior to starting to work on the study. Once training has been completed, staff must complete the Confirmation of Online Training Form to document what training has been completed. The trial team will also document attendance at SIVs. |
|
Is there a delegation log to complete? |
No. The Confirmation of Online Training Form will capture all trial training and will be used in place of a delegation log for this trial. |
|
One of the intervention arms is HFNO treatment, why is this included when this is currently not advised by several bodies due to the potentially harmful effects on HCW staff due to aerosolisation? |
We recognise the variability in international recommendations around use of HFNO, and hope that findings from this trial will help to inform future versions of recommendations with evidence as to the clinical effectiveness of HFNO. With regard to HFNO and aerosolisation, we agree that this, and CPAP, are aerosol generating, and if patients are receiving these treatments this should be in the setting of PPE usage.
However, if sites cannot, or do not wish to, provide HFNO, it is still possible to participate in the trial by enrolling to the standard care vs. CPAP arm, as our randomisation system accommodates for when only one of the interventions is available. |
|
Our site have raised concerns that oxygen flow will not be adequate (and even compromised) should these ‘high-flow’ devices be used excessively. We would appreciate your response to these concerns |
We are very much aware of the technical issues sites may have in general with the increase use of high flow devices, and are reiterating the advice circulated in the ICS statement around local sites ensuring close liaison between clinical and engineering teams on this issue, (https://www.ics.ac.uk/ICS/ICS/Pdfs/COVID-19/COVID-19_Safety_Alerts/Safety_Alert_High_Flow_Oxygen.aspx). |
|
Are extra consumables (e.g. masks) being provided? |
No separate consumables will be provided, an assessment will need to be done at site before randomisation to ensure device and relevant consumables are available. |
Screening |
|
|
Is screening limited to patients based on the intensive care unit? |
This is not limited just to patients based in ICU and can include other areas where the interventions are delivered. All patients admitted to hospital with suspected or confirmed COVID-19 can be screened for participation in the RECOVERY-RS trial. Site staff should liaise with and clinical teams across the hospital to identify patients. |
|
What screening information needs to be collected for the trial? |
Sites do not need to provide screening date to WCTU for the trial. To reduce the data burden on sites screening data will be collected centrally via the available national records. |
|
Is there a specific timescale from admission/meeting eligibility criteria to randomisation? |
There are no time restrictions, although this should be done as soon as possible. |
|
Can a patient previously recruited to the trial be recruited to the trial again if they are readmitted to hospital? |
No. |
Eligibility |
|
|
Inclusion criteria 3. FiO2 >0.4 and SpO2 <94%. Does the FiO2 >0.4 refer to oxygen delivered via a Venturi face mask, or can an approximate amount of oxygen be delivered by nasal cannulae? |
Either option is fine; patients who are potentially eligible for enrolment can be receiving oxygen via a Venturi face mask (in which case you may know the set FiO2) or an approximately similar amount via nasal cannulae (where only the flow rate is known). |
|
Inclusion criteria 3. FiO2 >0.4 and SpO2 <94%. Should patients meet this criteria for a certain duration to be eligible for the trial? |
Whilst a duration is not mandated only patients that require ongoing oxygenation should be entered into the trial. Patients whose oxygenation requirements are transient should not be considered eligible. |
|
As per inclusion criteria 4 who should determine whether a patient requires plan for escalation to intubation if needed? |
This decision should be made by the treating clinician.
|
|
For inclusion criteria 3. FiO2 >0.4 and SpO2 <94% does a time duration need to be satisfied? |
It is anticipated that by the time kit availability and other checks have been made a significant duration will have elapsed however this will remain a clinical decision to confirm a patient meets this eligibility criteria. |
|
Is radiology required prior to randomisation? |
No. Sites may do this as local practice, but it is not required. |
|
What If a patient is found to not have COVID after trial enrolment (i.e their tests come back negative)? |
The patient should remain in the trial and continue with their allocated treatment unless no longer clinically indicated. The patient will remain on study follow up and will be analysed on an intention to treat basis. This does not need to be recorded on the CRFs.
|
Receiving Consent |
|
|
What is the consent process for the trial?
|
England, Wales and Northern Ireland: If a patient has capacity they should be approached and consented prior to randomisation. If a patient does not have capacity they should be enrolled under emergency deferred consent process.
Scotland: If a patient has capacity they should be approached and consented prior to randomisation. If a patient does not have capacity they should not be enrolled. |
|
Is there a form (or similar) to complete for the patient notes with regard to the emergency waiver of consent? |
This should be captured in the patient notes, nothing further is mandated.
Note this does not apply to Scotland |
|
Can a personal consultee provide agreement over the telephone? |
Yes. Due to the visiting restrictions in place for COVID-19 patients, it is unlikely that a personal consultee will be physically available for consultation. Site teams may contact a personal consultee over the telephone and obtain verbal confirmation that they do not know of any objection to research participation expressed by the participant. The personal consultee can download the Patient Information Sheet from the trial website to read before making a decision.
Note this does not apply to Scotland |
|
If consent is received over the telephone, do we need to send a copy of the consent form to the patient/personal consultee? |
Yes, a copy of the signed consent form must be provided to the patient/personal consultee. This should be sent to them in accordance with local Trust policy and must comply with the Data Protection Act 2018. It may be emailed if local Trust policy allows and the patient/personal consultee has consented to provide their email address. Note this does not apply to Scotland |
|
Can participant capacity for consent purposes be assessed remotely? |
Participant capacity can be assessed by a member of the team remotely to allow sites to manage the risk of COVID-19 infection. |
|
Who can receive consent for study participation? |
Any member of the research team (e.g. doctors, nurses, research practitioners) can receive consent provided that: 1. The individual has completed the online consent training (and this is recorded) on the confirmation of online training, 2. This is permitted by local policy. |
|
Is there a timeframe for receiving consent following initial enrolment? |
We encourage sites to seek consent at the earliest and practical opportunity. In many cases, this will be after completion of any study intervention. |
Randomisation |
|
|
Can patients be enrolled into the study if one of the devices (CPAP and HFNO) are not available? |
Yes. If one of the devices is not available, or the site does not provide one of the interventions, then patients can still be enrolled into the trial. The randomisation system has been set up to allow randomisation to either CPAP vs standard care, or HFNO vs standard care. |
|
If a patient has previously received one of the interventions can they be considered for inclusion in the trial? |
Yes |
|
If a patient is currently receiving one of the interventions can they be considered for inclusion in the trial? |
No |
|
Who will be able to access the randomisation system and will there be a limit to the number of people who can access the system?
|
Randomisation is via an Interactive Voice Response (IVR) system (24/7 service). Call 02476 109940. PIN access to the randomisation IVRS is provided by email following ‘green light’ to begin recruitment. Each site will be provided with a generic username. Anyone who has undertaken the appropriate training (by attending an SIV or reading the randomisation slides on the training web page) and has completed a confirmation of training form can be provided with the generic username and IVRS PIN to enable them to randomise participants into the trial. |
|
If oxygen supplies are low at certain sites, could this mean disproportionate numbers of HFNO being not being randomised? |
If sites are unable to randomise due to oxygen supply, this is captured on the CRFs and will be appropriately considered in the statistical analysis plan. |
Co-enrolment |
|
|
Can patients be co-enrolled into other COVID-19 trials?
|
Co-enrolment will be reviewed on a case-by-case basis in accordance with NIHR-supported co-enrolment guidelines We will maintain a list of trials we are co-enrolling with on the trial website. |
Treatment |
|
|
How quickly should treatment be commenced in patients randomised to CPAP/ HFNO? |
A key purpose of the trial is that we are testing the early application of CPAP/ HFNO. The patient needs to be started on CPAP/ HFNO as soon as possible after randomisation meaning CPAP should not be delayed until the patient reaches a certain threshold in align with the protocol. |
|
Are both devices being classed as aerosol dispersing? |
We consider both CPAP and HFNO to be aerosol generating and recommend the use of appropriate PPE, in accordance with local policy |
|
Where a patient needs to be moved for CPAP/ HFNO (due to aerosol generation), should randomisation occur before or after moving patient? |
Randomisation should occur before moving as if the patient randomised to standard treatment, we would be exposing patient to transfer that is unnecessary and denying another patient access to an area where HFNO/CPAP can be delivered. |
|
For the CPAP settings – are either PEEP or EPAP acceptable? |
Yes |
|
What action should be taken if an eligible patient, who has escalation to intubation in their planned management at the time of enrolment, then has a change to their management plan? |
RECOVERY-RS is an intention to treat trial. The patient should not be withdrawn and treatment received should be recorded on the CRF as normal. |
|
Is crossover of treatment allowed? |
For patients in the oxygen therapy only, CPAP or HFNO should not be routinely used. We understand that there may be patient specific reasons why a clinician has to go off protocol and provide CPAP / HFNO. This should be on a case by case basis rather than a local protocol which puts all patients on CPAP/HFNO if the oxygen therapy only arm fails. The protocol guides that treatment failures should progress to intubation and invasive ventilation. |
|
If a patient does cross over between different treatments whilst on the trial how should this be recorded? |
Ideally this should be avoided wherever possible. However, the CRF allows for crossover of treatment to be captured by recording the amount of time of each intervention received. We will undertake additional statistical analyses to account for crossovers that occur during the trial. |
|
We don’t use the Venturi system therefore we do not have an accurate percentage of oxygen that patients require. Please could you advise if there is a conversion from litres to percent that you would like us to use? |
We do not recommend any specific conversion table so please use the table used for your site’s standard practice. It is also important to consider the overall clinical presentation of the patient as well. For example, if they are on 4LPM with a deteriorating respiratory status then it would be reasonable to consider them/discuss with clinical team whether the patient is for escalation of therapy and therefore appropriate to consider enrolling into the trial. |
Data collection |
|
|
How is cross-over of interventions documented? |
Cross-over of trial interventions will be recorded on the CRF. |
|
How is day 30 calculated?
|
Randomisation = day 0. Add 30 calendar days to calculate day 30. E.g. A participant randomised on May 1st. If they are not discharged prior to day 30 then day 30 would fall on May 31st. |
|
Data transfer: We don’t have nhs.net accounts, can we encrypt using another method? |
Yes. We are happy that sites follow local processes that adhere to appropriate Data Protection regulations. |
|
Discharge: What is the definition of discharge? |
For the purposes of the RECOVERY-RS trial is discharge from an Acute Care Hospital. |
|
Adverse events: Is there a specific definition for haemodynamic instability? |
No, clinical discretion to be used. |
|
Co-enrolment: which type of co-enrolment studies should be recorded on the CRF? |
Interventional and CTIMP studies should be included. There is no requirement to record observational studies. |
|
What is the case mix programme number required on the CRF? |
The case mix programme (CMP) is a dataset that is collected by ICNARC from ICU and HDU patients nationally, although there are sites who do not participate, particularly in the devolved nations. Patients who are admitted to ICU/HDU should be allocated an identifier which will link them to the CMP. By providing this patient identifier on the Data Collection CRF, the trial will be able to access the CMP dataset from ICNARC retrospectively. If a patient was not admitted to ICU/HDU they will not have a CMP number. If a patient does not have a CMP number, please indicate this on the Data Collection CRF by marking the data as unobtainable on the trial database - you should see a 'three dot' button on the right side of your screen, opposite this question which will allow you to mark the data as unobtainable and avoid future data queries. |
