Joseph Jones: Clathrin assembly and disassembly processes
If you have an enquiry related to a potential collaborative research project, please contact me via email: j.r.jones@warwick.ac.uk
A list of past and current collaborative projects can be found here.
Interdisciplinary PhD research topic:
Analysis and modelling of in vitro clathrin co-assembly processes
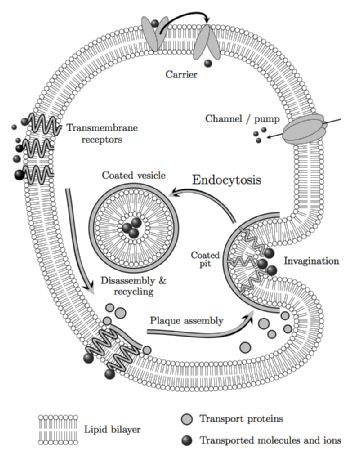
Clathrin mediated endocytosis is a fundamental cellular transport system that is integral to synaptic vesicle formation, receptor down-regulation and signalling, whereby eukaryotic cells select and uptake a variety of molecules for distribution to cellular compartments in a way that preserves the integrity of the membrane bilayer.
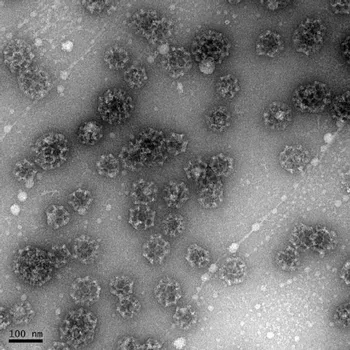
The clathrin coat complex is a complex adaptive structure that assembles to form vesicles of variable size according to the cargo and disassembles to allow recycling of the components. Aspects of this in vivo functionality are conserved when clathrin is isolated for study in vitro: e.g. by manipulation of the solvent acidity, clathrin triskelia assemble spontaneously to form heterogeneous, symmetrical, cage-like forms.
Clathrin is a molecule that has been studied since the late 1970s and investigation in to the disassembly properties of clathrin constructs has progressed by means of a `standard' kinetic assay (e.g. Bocking 2014, Rothnie 2011); yet the assembly properties of clathrin have been characterised only in fairly simple biochemical terms to date, using centrifugation assays in the main. Our project has focused on developing a methodology that now provides greatly improved, quantitative information for assembly.
The assembly and disassembly of supramolecular structures is a topic of great interest not only to life scientists who are interested in biological function but also to the physical sciences community who learn from cellular mechanisms to derive key principles of productive assembly for custom-made polymers and synthetic structures. Developing analytical tools to help better understand the cycle of co-assembly and disassembly exhibited by clathrin, as well as the effects of various accessory proteins, has wider relevance therefore and suggests there may be potential for the extension of novel methods in to many other applications.
Dynamic Light Scattering
 The remit for this EPSRC-funded research project has been to harness a physical science technique for solving a problem encountered in the biological sciences, using an integrated theoretical and experimental approach.
The remit for this EPSRC-funded research project has been to harness a physical science technique for solving a problem encountered in the biological sciences, using an integrated theoretical and experimental approach.
Over the course of this PhD project, we have developed a novel analysis of dynamic light scattering (DLS) measurements in conjunction with a set of experimental assays, which have now been optimised to study clathrin assembly. These assays provide useful options for experimental design, support the cross-validation of results and and have enabled us to identify a 'best' region of parameter space for collecting thermodynamic data and identifying statistically significant, reproducible effects in terms of the particle size distribution, e.g. due to addition of basic compounds, by co-assembly with a recombinant adaptor protein (and analysis of mutations), etc. We are now in a position to explore the wider application of our methodology, e.g. viral capsid assembly.
Using these methods, we have begun to address a fascinating question:
How do the characteristics of structural modularity and intra-component flexibility relate to clathrin's function at the nano-scale?
Home
References:
Bocking, T. et al. (2014), Key interactions for clathrin coat stability. Structure 22:1-11
Rothnie, A. et al. (2011), A sequential mechanism for clathrin cage disassembly by
70kDa heat-shock cognate protein (Hsc70) and auxilin. PNAS 108(17):6927-6932
