Synthetic methodology and organic synthesis
Our research is mainly based upon the use of free radicals in organic synthesis and polymer chemistry. We develop new methodology for mediating radical reactions with control of regio- and stereochemistry using green chemistry. We have developed a range of copper based catalysts to facilitate C-C bond formation in cyclisation reactions. We also have an interest in the chemistry of hydroxamic acids and organozirconium chemistry.
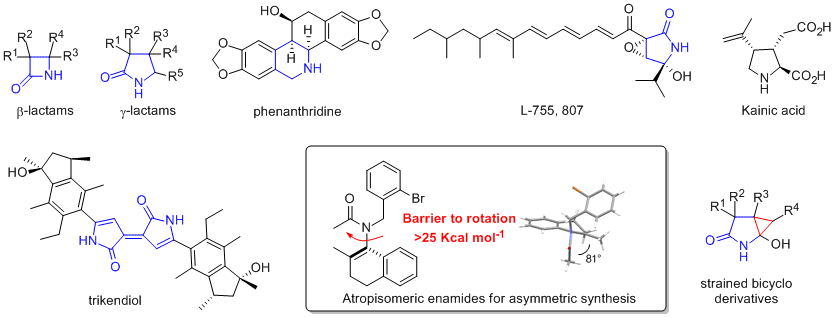
Targets: Heterocycles, atropisomeric enamides, beta-lactams, gamma-lactams, phenanthridine alkaloids, L-755807, trikendiol, kainic acids, indolizidines, oxindoles and strained bicyclo gamma-lactam derivatives. For a publication list see here:
For more specific examples of our research see:
Copper catalysed atom transfer radical cyclisation. Key paper.
CuSO4.5H2O in C/C bond formation. Key paper.
Catalysts for atom transfer radical cyclisation. Key paper
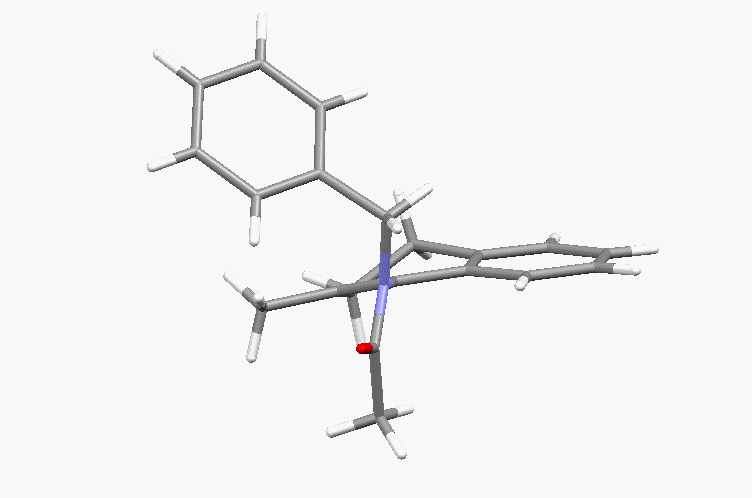
Atropisomeric enamides and their use in asymmetric chemistry. Key paper
Beta-lactam synthesis. Key paper.
Solid supported reagents for radical reactions. Key paper.
Novel 5-endo radical-polar crossover reactions. Key paper.
Amidyl Radical Cyclisations.Key Paper.
Novel triple crossover reactions to trikendiol skeleton. Coming soon-hopefully!
Collaborators
Prof. Mark Smith
Dr. David Fox
Prof. Robert Deeth
Sponsors
AstraZeneca
Lilly UK
Aventis
Roche
Knoll Pharmaceuticals
EPSRC
