Szymon Abrahamczyk
Currently, a student at the University of Warwick PhD Analytical Science. A member of Analytical Science Centre for Doctoral Training since September 2019 and the Hatton group since September 2020. The main interest is in energy materials, Organic Photovoltaics (OPV). My PhD project is regarding Low-work, air-stable electrodes for OPV applications.
Publications:
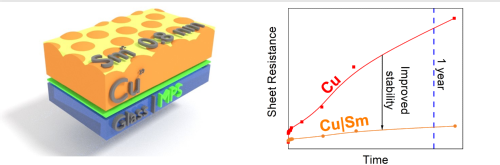
Passivating polycrystalline copper with an ultra-thin samarium layer
Szymon Abrahamczyk, Marc Walker, Yisong Han, Steven Huband, David Walker and Ross A.Hatton
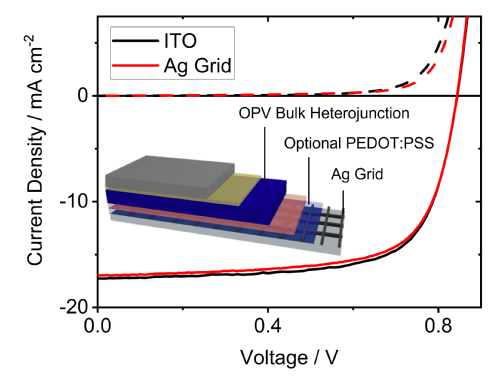
Philip Bellchambers, Charlie Henderson, Szymon Abrahamczyk, Seungsoo Choi, Jin-Kyun Lee, Ross A. Hatton
Design rules for selective deposition of silver by condensation coefficient modulation for application in organic photovoltaics
Presented at:
- Oral talk: 16th International Symposium on Flexible Organic Electronics in Thessaloniki, Greece (3rd - 7th Jul)
Plasmonic metal electrodes with sub-wavelength nanofeatures fabricated by condensation coefficient modulation
Presented at:
-
Poster and Oral presentation: AS CDT Annual Conference (13th - 16th Jul 2022)
-
Poster: 14th International Conference on Hybrid and Organic Photovoltaics (23rd - 25th May 2022)
Passivating polycrystalline copper with an evaporated ultra-thin samarium layer
Presented at:
- Oral presentation: European Materials Research Society Spring Meeting 2022 (30th May - 3rd Jun 2022)
- Poster: 16th Photovoltaic Science, Applications and Technology Conference (6th - 8th Apr 2022)
- Poster: 5th RSC Energy Sector Early Careers Event (23rd Nov 2021)
- Poster: AS CDT Annual Conference (6th - 9th Sep 2021)
Conference attendance
- AS CDT Annual Conference 2022 - Poster and oral presentation “Plasmonic metal electrodes with sub-wavelength nanofeatures fabricated by condensation coefficient modulation”
- E-MRS Spring Meeting 2022 - Oral presentation “Plasmonic metal electrodes with sub-wavelength nanofeatures fabricated by condensation coefficient modulation”
- HOPV22 - Poster presentation: “Plasmonic metal electrodes with sub-wavelength nanofeatures fabricated by condensation coefficient modulation”
- PVSAT16 2022 - Poster presentation: “Passivating polycrystalline copper with samarium oxide for use as a reflective cathode in organic photovoltaics.”
- 5th RSC Energy Sector Early Careers Event 2021 - Poster presentation: “ Passivating polycrystalline copper with samarium oxide for use as a reflective cathode in organic photovoltaics.”
- HOPV21 - Attendance
- AS CDT Annual Conference 2021 - Poster and video presentation “Characterisation of copper electrode passivated with samarium oxide overlayer”
- AS CDT Annual Conference 2020 - Flash presentation ”Thin metal films with nanoholes for plasmonic sensors”
Education:
2020 - now PhD Analytical Science (University of Warwick)
Project: "Characterisation of low work function, air-stable electrodes for organic photovoltaics." Supervised by prof. Ross A. Hatton
2019 - 2020 MSc Analytical Science (University of Warwick) - First class with Distinction
Project 1: “Ultrathin metal film sensors.” Supervised by Dr Ross A. Hatton
Project 2: “Investigation of the mechanism of passivation on TFSI-enhanced SiO2 layers on silicon wafers using x-ray photoelectron spectroscopy.” Supervised by Dr John Murphy
2016 - 2019 BSc Analytical Chemistry and Forensic Science (Coventry University)
Final year project: “Sonochemically activated persulfate induced degradation of traces of tetracyclines in water samples" Supervised by Dr Larysa Paniwnyk