Information for Site Staff
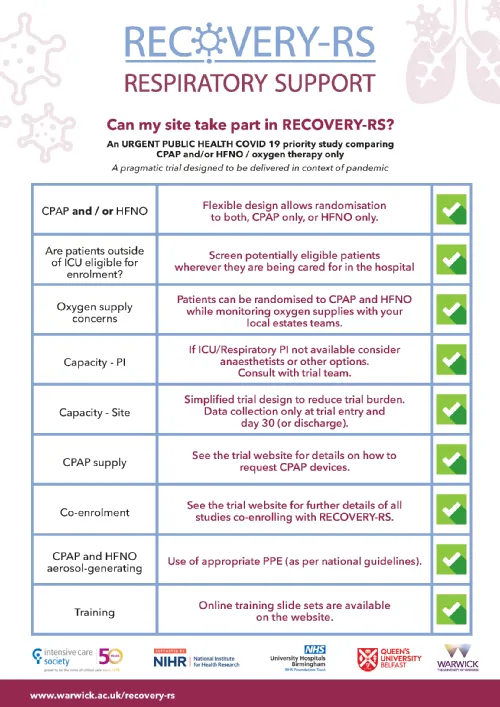
There are currently no approved treatments for COVID-19. This trial will assess the effects of different non-invasive ventilation options for adults with suspected or confirmed COVID-19. All patients will receive the usual care from the participating hospitals.
RECOVERY-RS is a randomised trial and every COVID-19 patient in England, Wales and Northern Ireland over the age of 18 years may be invited to participate. Randomisation will be to one of three arms: Continuous Positive Airway Pressure (CPAP); High-flow Nasal Oxygen (HFNO) or standard care (excluding CPAP and HFNO). The trial is designed to have the least possible impact on NHS staff.
Everything you need to know about setting up RECOVERY-RS at your site:
Trial documentation
(All site set up and trial documentation can be found on this page).
Training
Randomisation
Follow-up
Co-Enrolment
Recruitment Tracker
Frequently Asked Questions
Trial Supplies
Safety Alert: High-flow oxygen devices, including real-world incident
Issued: Sunday 5 April, 2020
NHSEI issued an Urgent Patient Safety Notice regarding the “Use of high flow oxygen therapy devices (including wall CPAP, and high flow face mask or nasal oxygen) during the Coronavirus epidemic” on 31 March 2020 (NHSE/I – 2020/001). The alert required immediate action from all hospitals to mitigate the risk posed by the use of multiple high flow devices, and highlighted “If the demand through multiple wall outlets exceeds the maximum capacity of the VIE delivery system, there is a risk of a rapid pressure drop in oxygen supply pipes. This could lead to a failure of oxygen delivery systems throughout the hospital, including to patients on face masks, CPAP, ventilators and operating theatres. There is also a risk of rapid and unpredictable depletion of the VIE. Both of these situations present a potentially significant risk to multiple patients simultaneously.”
The ICS is aware of multiple hospitals that are currently experiencing significant burden on their oxygen infrastructure, and that in particular a major incident occurred at an East of England hospital yesterday due to compromise of their oxygen infrastructure. This was preceded by low-pressure alarms (as seen at other sites) and in an urgent need to evacuate ICU patients to nearby NHS Trusts.
We also know that several hospitals have tested their oxygen infrastructure and found their ability to use multiple high-flow devices simultaneously is less than expected given their VIE capacity and pipework.
We strongly encourage all institutions to pay close attention to the actions required in the UPSN, specifically:
Urgently ensure liaison between clinicians and hospital oxygen engineering teams to ascertain:
- the maximum flow rate from your VIE
- any additional limitations to oxygen delivery owing to pipework architecture
- calculate the maximum number of patients who can be treated with high flow devices such as wall CPAP and communication of this to the relevant clinical teams
- undertake a daily count of the number of high-flow systems where the potential oxygen flow rate exceeds 10 L/min (this will include most wall CPAP systems, High Flow Nasal Oxygen (HFNO), some non-invasive ventilators used in acute settings, and many ventilators used in critical care units or operating theatres).
- implement safety measures to prevent accidental O2 system failure (such as limiting the number of these devices available for clinical use).
In addition, we wish to highlight that should your hospital oxygen system develop “low oxygen pressure alarms” in the context of high-flow device usage, this is likely to be an early indication of approaching flow limitation and urgent action should be taken to review the usage of these devices, reduce demand on the oxygen infrastructure, and urgently escalate for oxygen engineering assistance.
With well wishes
Dr Ganesh Suntharalingam
President, Intensive Care Society
Trial Protocol
Inclusion/Exclusion Criteria
Trial Participant Checklist
Consenting patient prior to treatment
Consent form (on commencement)
Patient Information Leaflet (on commencement)
Short Patient Information Leaflet (on commencement)
Easy Read PIS (print)
Easy Read PIS (screen)
Deferred consent
Patient Information Sheet
Short Patient Information Leaflet
Consultee Declaration Form & Cover Letter
Deferred Consent Form