Covid-19 & EUTOPIA: Future targets for treatments rapidly identified with new computer
- Researchers at the University of Warwick and EUTOPIA model movements of nearly 300 protein structures in Covid-19
- Scientists can use the simulations to identify potential targets to test with existing drugs, and even check effectiveness with future Covid variants
- Simulation of virus spike protein, part of the virus’s ‘corona’, shows promising mechanism that could potentially be blocked
- Researchers have publicly released data on all protein structures to aid efforts to find potential drug targets: https://warwick.ac.uk/flex-covid19-data
- Images and video available, see Notes to Editors
 Researchers have detailed a mechanism in the distinctive corona of Covid-19 that could help scientists to rapidly find new treatments for the virus, and quickly test whether existing treatments are likely to work with mutated versions as they develop.
Researchers have detailed a mechanism in the distinctive corona of Covid-19 that could help scientists to rapidly find new treatments for the virus, and quickly test whether existing treatments are likely to work with mutated versions as they develop.
The team, led by the University of Warwick as part of the EUTOPIA community of European universities, have simulated movements in nearly 300 protein structures of the Covid-19 virus spike protein by using computational modelling techniques, in an effort to help identify promising drug targets for the virus.
In a new paper published today (19 February) in the journal Scientific Reports, the team of physicists and life scientists detail the methods they used to model the flexibility and dynamics of all 287 protein structures for the Covid-19 virus, also known as SARS-CoV-2, identified so far. Just like organisms, viruses are composed of proteins, large biomolecules that perform a variety of functions. The scientists believe that one method for treating the virus could be interfering with the mobility of those proteins.
They have made their data, movies and structural information, detailing how the proteins move and how they deform, for all 287 protein structures for Covid-19 that were available at the time of the study, publicly accessible to allow others to investigate potential avenues for treatments.
The researchers focused particular efforts on a part of the virus known as the spike protein, also called the Covid-19 echo domain structure, which forms the extended corona that gives coronaviruses their name. This spike is what allows the virus to attach itself to the ACE2 enzyme in human cell membranes, through which it causes the symptoms of Covid-19.
The spike protein is in fact a homotrimer, or three of the same type of protein combined. By modelling the movements of the proteins in the spike, the researchers identified a ‘hinge’ mechanism that allows the spike to hook onto a cell, and also opens up a tunnel in the virus that is a likely means of delivering the infection to the hooked cell. The scientists suggest that by finding a suitable molecule to block the mechanism – literally, by inserting a suitably sized and shaped molecule – pharmaceutical scientists will be able to quickly identify existing drugs that could be effective against the virus.
Lead author Professor Rudolf Roemer from the Department of Physics at the University of Warwick, who conducted the work while on a sabbatical at CY Cergy-Paris Université, said: “Knowing how this mechanism works is one way in which you can stop the virus, and in our study we are the first to see the detailed movement of opening. Now that you know what the range of this movement is, you can figure out what can block it.
“All those people who are interested in checking whether the protein structures in the virus could be drug targets should be able to examine this and see if the dynamics that we compute are useful to them.
“We couldn’t look closely at all the 287 proteins though in the time available. People should use the motion that we observe as a starting point for their own development of drug targets. If you find an interesting motion for a particular protein structure in our data, you can use that as the basis for further modelling or experimental studies.”
On his connection to the EUTOPIA community, Professor Roemer said:
"This project was conceived while I was a Fellow in Residence at CY Cergy Paris Université, where I worked on machine learning with an interdisciplinary research group from across Europe in the Advanced Studies centre.
"The opportunity to collaborate with European academic colleagues in a major science hub in Paris was made possible thanks to Warwick being a member of the EUTOPIA network. I am excited about the further shared ideas and innovations that deeper collaboration and mobility across the continent will bring."
To investigate the proteins’ movements, the scientists used a protein flexibility modelling approach. This involves recreating the protein structure as a computer model then simulating how that structure would move by treating the protein as a material consisting of solid and elastic subunits, with possible movement of these subunits defined by chemical bonds. The method has been shown to be particularly efficient and accurate when applied to large proteins such as the coronavirus’s spike protein. This can allow scientists to swiftly identify promising targets for drugs for further investigation.
The protein structures that the researchers based their modelling on are all contained in the Protein Data Bank. Anyone who publishes a biological structure has to submit it to the protein databank so that it is freely available in a standard format for others to download and study further. Since the start of the Covid-19 pandemic, scientists all over the world have already submitted thousands of protein structures of Covid-19-related proteins onto the Protein Data Bank.
Professor Roemer adds: “The gold standard in modelling protein dynamics computationally is a method called molecular dynamics. Unfortunately, this method can become very time consuming particularly for large proteins such as the Covid-19 spike, which has nearly 3000 residues – the basic building blocks of all proteins. Our method is much quicker, but naturally we have to make more stringent simplifying assumptions. Nevertheless, we can rapidly simulate structures that are much larger than what alternative methods can do.
“At the moment, no-one has published experiments that identify protein crystal structures for the new variants of Covid-19. If new structures come out for the mutations in the virus then scientists could quickly test existing treatments and see if the new mechanics have an impact on their effectiveness using our method.”
The University of Warwick is a founding member of EUTOPIA, a network of six European universities working together to create a new model for higher education across the continent – one that is based on increased mobility, inclusivity, and serving its six regional communities.
EUTOPIA has invested in co-teaching PhD and MA scholars between different European countries, as well as continuing to establish a growing number of academic and professional collaborations between the UK, France, Belgium, Spain, Sweden, and Slovenia, with the aim of tackling real-world challenges. In 2019, EUTOPIA was included in the prestigious ERASMUS+ ‘European Universities’ programme. More information here.

- All data, simulations and videos for the protein structures referenced in this research are publicly available online via this link: https://warwick.ac.uk/flex-covid19-data
- ‘Flexibility and mobility of SARS-CoV-2-related protein structures’ will be published in Scientific Reports, DOI: 10.1038/s41598-021-82849-2 Link: www.nature.com/articles/s41598-021-82849-2 (to go live after the embargo)
Ends
Notes to editors:
Images and video available to download:
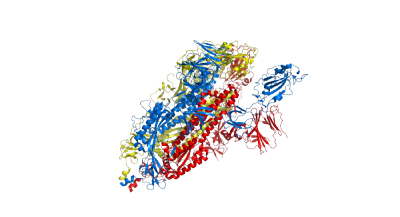
Top and side views of spike protein structure 6vyb. Colors blue, red and yellow denote the 3 sub-parts of the homodimer:
Snapshots of spike protein structure 6vyb opening and closing. The 3 small images show the crystal as well as the most closed and most opened conformation of the spike during its movement. The central, large image is a superposition of all 3 conformations:
Videos of spike protein structure 6vyb opening and closing:
Snapshots of spike protein structure 6vxx, a closed structure which stays closed. Colours and presentation as for the structure 6vyb:
19 February 2021
For further information contact:
Luke Walton, International Press Manager
+44 (0) 7823 362 150
or
Peter Thorley
Media Relations Manager (Warwick Medical School and Department of Physics) | Press & Media Relations | University of Warwick
Email: peter.thorley@warwick.ac.uk
Mob: +44 (0) 7824 540863
